Question
Question: From the following data, calculate the enthalpy change for the combustion of cyclopropane at 298K. T...
From the following data, calculate the enthalpy change for the combustion of cyclopropane at 298K. The enthalpy of formation of CO2(g), H2O(l) and propene(g) are -393.5,-285.8 and 20.42kJmol−1 respectively. The enthalpy of isomerisation of cyclopropane to propene is -33.0kJmol−1.
Solution
The answer for this question is based on the simple calculation using the formula of enthalpy that is the summation of enthalpies of the whole balanced reaction that is taking place according to Hess law.
Complete step – by – step solution:
In our lower classes of physical chemistry part, we have come across the thermodynamics which includes several parameters for the reaction which is undergoing.
Let us understand the basic concept regarding thermodynamics that tells us how to calculate the enthalpy change in a balanced chemical equation.
Here, enthalpy change is nothing but the change in the amount of heat absorbed or evolved in a reaction which is usually carried out at constant pressure.
Now, in the above question, the reactant is the cyclopropane which undergoes a combustion reaction to produce carbon dioxide and water.
The balance equation for this can be written as shown below,
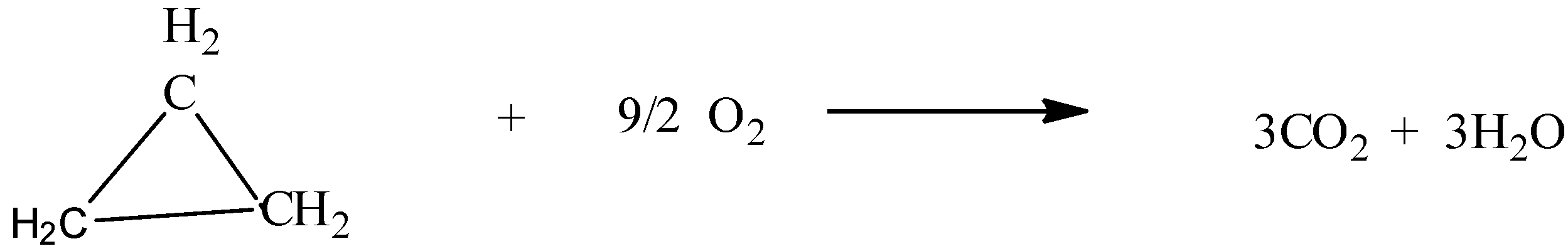
Now, the following data given in the question is as follows,
Enthalpy change for the formation ofCO2is -393.5kJmole−1 and let us denote this as ΔH1=−393kJ/mol
Reaction isC+O2→CO2
Similarly for oxygen, ΔH2=−285.8kJ/moland the reaction is,
H2+21O2→H2O
For the isomer propene, the enthalpy is ΔH3=20.42kJ/mol
The reaction here is,
2C+2H2→C2H4
And ΔHO2=0
Now, the enthalpy change for isomerisation of cyclopropane to propene is given by, ΔH4=−33.0kJ/mol (Δ1) and reaction is given by,
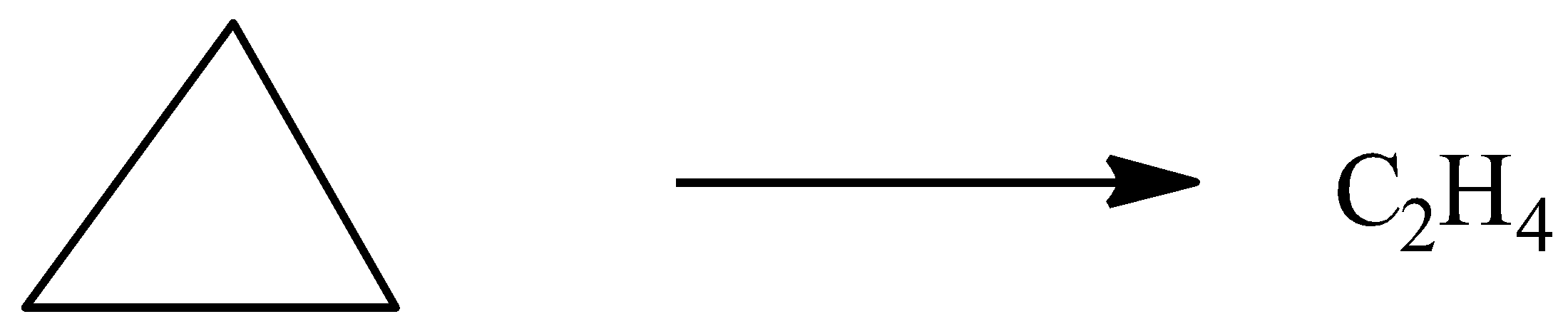
Now, based on the main reaction, we can combine the enthalpies accordingly with balanced equation and Hess law as,
ΔH=ΔH4+[3×ΔH1+3×ΔH2−ΔH3−29−ΔHO2]
⇒ΔH=−33[3×(−393.5)+3×(−285.8)−20.42−29(0)]
⇒ΔH=−2091.32kJ/mol
Therefore, the correct answer is ΔH=−2091.32kJ/mol
Note: Whenever a question relating to thermodynamics is asked where the reaction is not given but just data, make sure that you write a balanced equation prior solving the internal energy for the reaction.
