Question
Question: From the following compounds choose the one which is not aromatic? A. 
B.
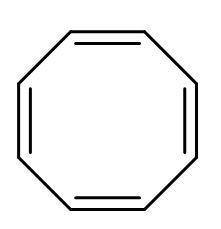
C.
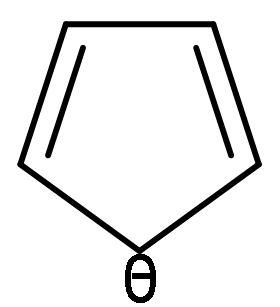
D.
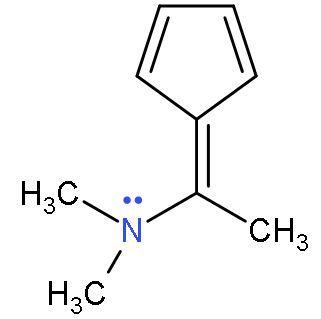
Solution
Not aromatic or aromatic compounds can be checked by certain rules, if it follows then, aromatic and if not then, not aromatic. Aromaticity is a special property which is seen in organic compounds of ring-shaped, flat structures with a ring of conjugated systems that gives increased stability.
Complete step by step answer:
An aromatic compound contains a set of covalent bonds with specific characteristics:
(1) A delocalized conjugated system of π electrons, which is an arrangement of alternating single and double bonds.
(2) Should be a coplanar structure having all the atoms in the same plane.
(3) Should follow Huckel’s rule, a number of π delocalized electrons, (4n + 2) π-electrons, where n = 0, 1, 2, 3, and so on.
(4) Atoms should be arranged in one or more rings.
Let us now see the compounds one by one to check whether they are aromatic or not.
| Options | Structures | Follows rule (1) i.e. conjugates system | Follows rule (2) i.e. planarity | Follows rule (3) i.e. Huckel’s rule | Follows rule (4) i.e. cyclic structure | Aromatic or not aromatic |
|---|---|---|---|---|---|---|
| A. | 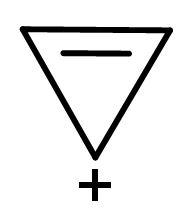 | The electrons of double bonds are in resonance with the positive charge, so it is a conjugated system. | All the carbon atoms are sp2 hybridized. Due to double bond and positive charge. It is planar. | The compound has 2 π-electrons. If we put n=0 in (4n + 2) π-electrons, then, it gives 2 π-electrons. Hence, follows Huckel’s rule. | It is a cyclic structure. As, all π-electrons are inside the ring. | Follows all the rules, hence aromatic. |
| B. | 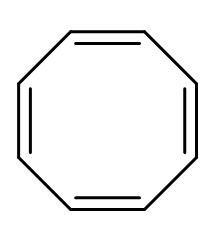 | The electrons of double bonds are in resonance with each other, so it is a conjugated system. | All the carbon atoms are sp2 hybridized. Due to double bond and positive charge. It is planar. | The compound has 8 π-electrons. For no value of n, the rule is not satisfied. | It is a cyclic structure. As, all π-electrons are inside the ring. | Does not follow Huckel’s rule, hence not aromatic. |
| C. | 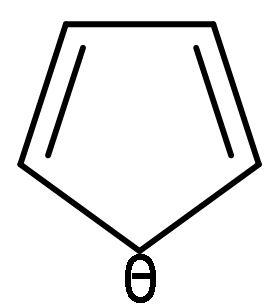 | The electrons of double bonds are in resonance with the negative charge, so it is a conjugated system. | All the carbon atoms are sp2 hybridized. Due to double bond and positive charge. It is planar. | The compound has 6 π-electrons. If we put n=1 in (4n + 2) π-electrons, then, it gives 6 π-electrons. Hence, follows Huckel’s rule. | It is a cyclic structure. As, all π-electrons are inside the ring. | Follows all the rules, hence aromatic. |
| D. | 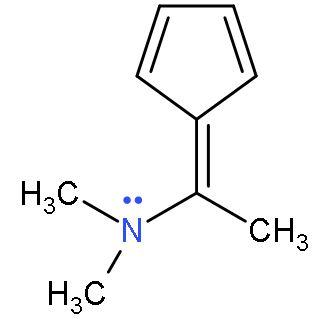 | The ring system does not seem to follow the conjugated system. But, the lone pair of the nitrogen is in conjugation with double bond. The resonating structure is 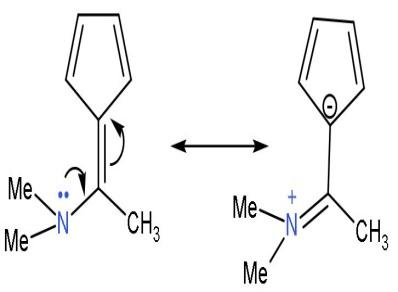 On resonance, it forms one carbanion inside the 5-membered ring with 2 double bonds and a negative charge, so it is a conjugated system. On resonance, it forms one carbanion inside the 5-membered ring with 2 double bonds and a negative charge, so it is a conjugated system. | All the carbon atoms are sp2 hybridized (considering the aromatic part or ring system). Due to double bond and positive charge. It is planar. | The compound has 6 π-electrons. If we put n=1 in (4n + 2) π-electrons, then, it gives 6 π-electrons. Hence, follows Huckel’s rule. | It is a cyclic structure. As, all π-electrons are inside the ring. | Follows all the rules, hence aromatic. |
So, the correct answer is “Option B”.
Note: The compound ‘d’ does not seem to be aromatic by its structure because the ring has 4 π-electrons, which does not satisfy Huckel’s rule. But on resonance, with other atoms, a resonating structure is formed which is aromatic. So, do not judge aromaticity by just looking at the compound, it can be aromatic in one form or another.
