Question
Question: From the following bond energies: \( H - H \) bond energy: 431.37KJmol−1431.37KJmol-1 \( C = ...
From the following bond energies:
H−H bond energy: 431.37KJmol−1431.37KJmol-1
C=C bond energy: 606.10KJmol−1606.10KJmol-1
C−C bond energy: 336.49KJmol−1336.49KJmol-1
C−H bond energy: 410.50KJmol−1410.50KJmol-1
Enthalpy for the reaction
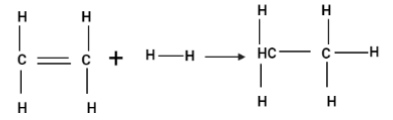
will be:
(A) −243.6KJ.mol−1
(B) −120KJ.mol−1
(C) 553KJ.mol−1
(D) 1523.6KJ.mol−1
Solution
Hint : The change in the enthalpy of a chemical reaction that happens at constant pressure is referred to as the Heat of Reaction (also known as Enthalpy of Reaction). It's a thermodynamic unit of measurement that can be used to calculate the amount of energy released or emitted per mole in a reaction.
Complete Step By Step Answer:
We know that the net enthalpy is the difference between the summation of enthalpy of reactants and the summation of enthalpy of product.
ΔH=∑HR−∑Hp
Now we will calculate the reactant and product enthalpy separately by counting the number of bonds and the types of bonds each reactant and products have.
\sum {{H_R}} = 1(C = C) + 4(C - H) + 1(H - H) \\\
\sum {{H_R}} = 606.10 + 4(410.50) + 1(431.37) \\\
\sum {{H_R}} = 606.10 + 1642 + 431.37 \\\
\sum {{H_R}} = 2679.47 \\\
\sum {{H_p}} = 6(C - H) + 1(C - C) \\\
\sum {{H_p}} = 6(410.50) + 1(336.49) \\\
\sum {{H_p}} = 2463 + 336.49 \\\
\sum {{H_p}} = 2799.49 \\\
Hence, the net enthalpy is
=2679.47−2799.49 =−120.02KJ.mol−1
Therefore, option (B) is the correct option.
Note :
The enthalpy of any substance rises with temperature, which ensures that the enthalpies of both the components and the reactants rise. If the enthalpies of the products and reactants escalate at various rates, the reaction's total enthalpy can change.
