Question
Question: From symbol \(_{16}{S^{32}}\) (Sulphur) state: i) Atomic number of Sulphur ii) Mass number of Su...
From symbol 16S32 (Sulphur) state:
i) Atomic number of Sulphur
ii) Mass number of Sulphur
iii) Electronic configuration of Sulphur
Solution
Sulfur is found in the group 16 of the periodic table. It is a non-metal and is obtained as a byproduct after the production of natural gas. It is generally bright yellow in color and it has an extremely bad odor similar to that of rotten eggs.
Complete step by step answer:
Sulfur is basically a multivalent non-metal which is tasteless and odorless. It is a yellow crystalline solid in its native form. It is a chemical element with symbol S and atomic number 16. Moreover, under normal conditions, the sulfur atoms form cyclic octatomic molecules with the chemical formula S8. It is mostly found near hot springs and volcanoes. Now, let us discuss some of the allotropes of sulfur. The first one is yellow rhombic Sulphur i.e. α sulfur and the other one is monoclinic sulfur i.e. β sulfur.
Now, rhombic sulfur is crystalline in nature and has an octahedral shape. We get rhombic sulfur by heating the solution of rolled sulfur in CS2 whereas monoclinic sulfur is obtained by cooling the melted rhombic sulfur in the dish. Both the forms of sulfur are as shown:
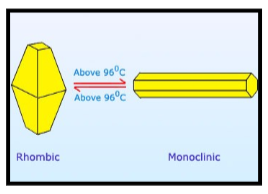
Now, let’s discuss each subpart given in the question.
i) The atomic number of sulfurs is16
ii) Mass number of sulfurs is 32
iii) Electronic configuration is [Ne]3s23p4
Note: Basically, sulfur has gained its importance because of its uses. Its uses are not just limited to the industries but it also plays a vital role in our ecosystem by affecting the growth of the plants. This has further led to the development of many sulfur-containing fertilizers.
