Question
Question: Friedel-Craft’s reaction using \(MeCl\) and anhydrous \(AlC{l_3}\) will take place most efficiently ...
Friedel-Craft’s reaction using MeCl and anhydrous AlCl3 will take place most efficiently with:
(A) benzene
(B) nitrobenzene
(C) acetophenone
(D) toluene
Solution
Friedel-Craft’s is an electrophilic aromatic substitution reaction in which a carbocation attacks on an aromatic ring.
There are two primary friedel-Crafts reactions i.e., alkylation and acylation.
Example:
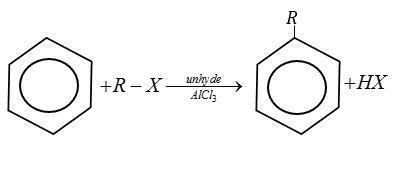
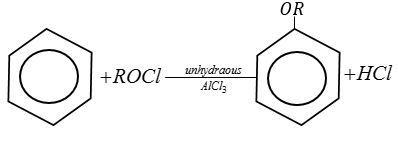
Complete step by step answer:
Let us write Friedel-Crafts reactions one by one with the given option.
Diagram:
It takes place at a slower rate.
Friedel-Crafts reaction with acetophenone takes place at high pressure and not possible.
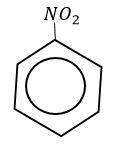
With nitrobenzene it is not possible because electrons are a withdrawing group present in the benzene ring.
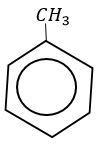
With toluene it takes place at a faster rate due to hyper conjugation.
Hyper conjugation is interaction of electrons in σ-bond (usually C-H or C-C) with an adjacent empty or partially filled P-orbital or π-orbital.
This increases the stability of molecules.
This is also known as no-bond.
This is a permanent effect.
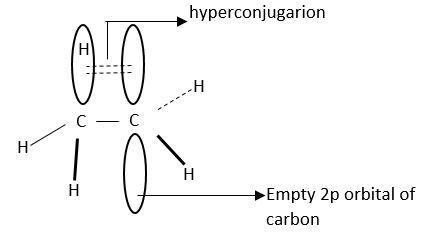
Hyperconjugation stabilizes carbocation as it helps in dispersal of positive charges.
As the number of α−H−atoms increases. The relative stability on the basis of hyperconjugation increase
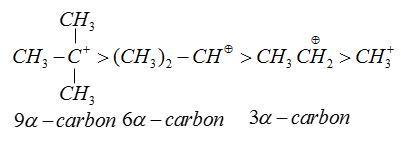
Therefore, form the above explanation the correct option toluene. 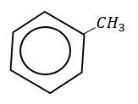
So, the correct answer is “Option D”.
Note:
Friedel-Crafts reactions always occur in the presence of anhydrous AlCl3.Anhydrous AlCl3 converts nucleophile and electrophile.
In Aromatic compounds only electrophilic substitution takes place.
