Question
Question: $\frac{Br_2}{2} + OH^{\ominus} \longrightarrow Br^{\ominus} + BrO_3^{\ominus} + n_2O$ (Hot conc.) r...
2Br2+OH⊖⟶Br⊖+BrO3⊖+n2O (Hot conc.)
red"
nred=2∣−1−0∣=2
noxidn=2∣5−0∣=10
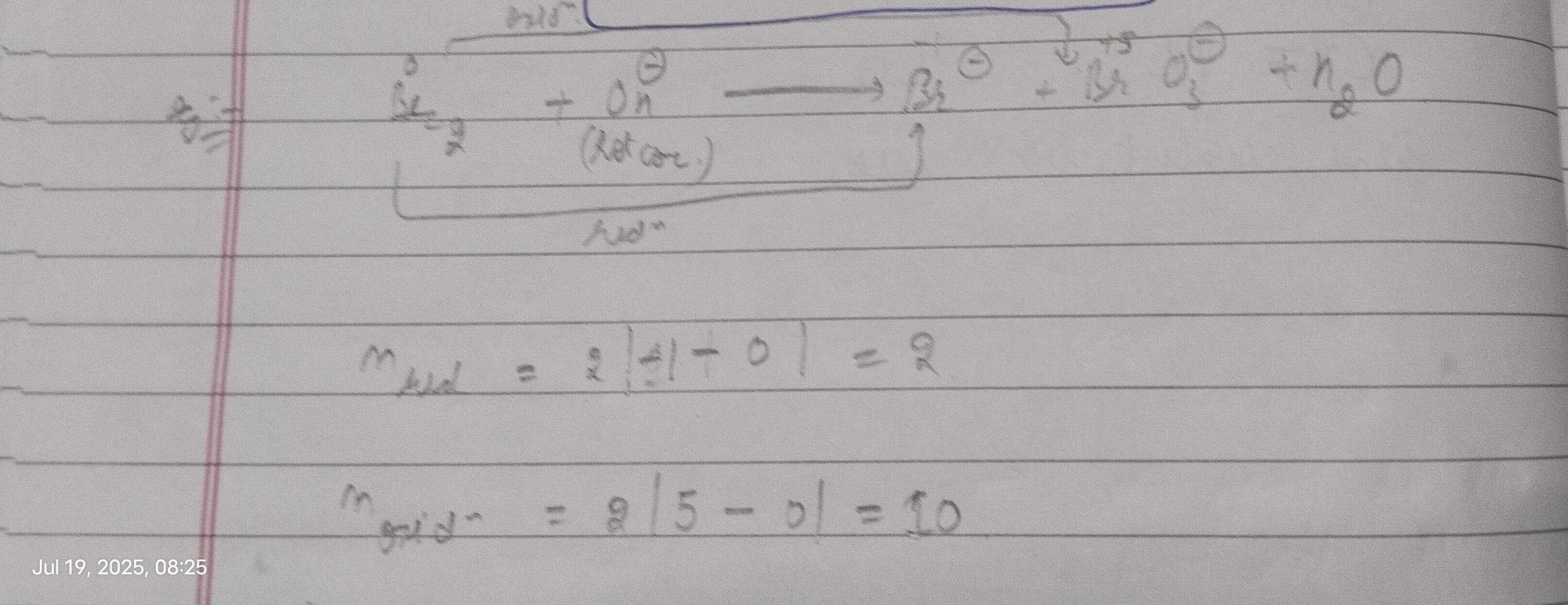
3Br_2 + 6OH^{\ominus} \longrightarrow 5Br^{\ominus} + BrO_3^{\ominus} + 3H_2O
Solution
The given reaction is a disproportionation reaction of Bromine in a hot concentrated hydroxide solution. Bromine (oxidation state 0) is simultaneously reduced to Bromide (oxidation state -1) and oxidized to Bromate (oxidation state +5).
The provided calculations are for the number of electrons transferred per Br2 molecule in each half-reaction:
-
Reduction half-reaction: Br2⟶Br⊖
- Oxidation state of Br changes from 0 to -1.
- For one Br atom, the change is ∣−1−0∣=1.
- Since Br2 molecule contains 2 Br atoms, the total number of electrons gained (nred) by one Br2 molecule is 2×∣−1−0∣=2×1=2.
- This corresponds to the provided nred=2∣−1−0∣=2.
-
Oxidation half-reaction: Br2⟶BrO3⊖
- Oxidation state of Br changes from 0 to +5.
- For one Br atom, the change is ∣+5−0∣=5.
- Since Br2 molecule contains 2 Br atoms, the total number of electrons lost (noxidn) by one Br2 molecule is 2×∣+5−0∣=2×5=10.
- This corresponds to the provided noxidn=2∣5−0∣=10.
These values are crucial for balancing the redox reaction using the oxidation state method or ion-electron method. The number of electrons gained must equal the number of electrons lost.
Balancing the reaction using the Ion-Electron Method (in basic medium):
Step 1: Write the unbalanced half-reactions. Reduction: Br2⟶Br⊖ Oxidation: Br2⟶BrO3⊖
Step 2: Balance atoms other than O and H. Reduction: Br2⟶2Br⊖ Oxidation: Br2⟶2BrO3⊖
Step 3: Balance O atoms by adding H2O and H atoms by adding H⊕. Then neutralize H⊕ with OH⊖ for basic medium.
- Reduction half-reaction: No O or H atoms. Br2⟶2Br⊖
- Oxidation half-reaction: Br2⟶2BrO3⊖ (6 oxygen atoms on RHS) Balance O by adding H2O: Br2+6H2O⟶2BrO3⊖ (12 H atoms on LHS) Balance H by adding H⊕: Br2+6H2O⟶2BrO3⊖+12H⊕ Since the reaction is in basic medium, add 12OH⊖ to both sides to neutralize H⊕: Br2+6H2O+12OH⊖⟶2BrO3⊖+12H⊕+12OH⊖ Combine H⊕ and OH⊖ to form H2O: Br2+6H2O+12OH⊖⟶2BrO3⊖+12H2O Cancel H2O molecules: Br2+12OH⊖⟶2BrO3⊖+6H2O
Step 4: Balance charges by adding electrons.
- Reduction half-reaction: Br2+2e⊖⟶2Br⊖ (Charge: 0 on LHS, -2 on RHS. Add 2 electrons to LHS)
- Oxidation half-reaction: Br2+12OH⊖⟶2BrO3⊖+6H2O (Charge: -12 on LHS, -2 on RHS. Add 10 electrons to RHS) Br2+12OH⊖⟶2BrO3⊖+6H2O+10e⊖
Step 5: Make the number of electrons equal in both half-reactions. Multiply the reduction half-reaction by 5 and the oxidation half-reaction by 1 (to get 10 electrons on both sides). Reduction: 5(Br2+2e⊖⟶2Br⊖)⟹5Br2+10e⊖⟶10Br⊖ Oxidation: 1(Br2+12OH⊖⟶2BrO3⊖+6H2O+10e⊖)⟹Br2+12OH⊖⟶2BrO3⊖+6H2O+10e⊖
Step 6: Add the balanced half-reactions and simplify. 5Br2+10e⊖⟶10Br⊖ Br2+12OH⊖⟶2BrO3⊖+6H2O+10e⊖
6Br2+12OH⊖⟶10Br⊖+2BrO3⊖+6H2O
Divide all coefficients by 2 to get the simplest integer coefficients: 3Br2+6OH⊖⟶5Br⊖+BrO3⊖+3H2O
The balanced chemical equation is: 3Br2+6OH⊖⟶5Br⊖+BrO3⊖+3H2O
The provided values nred=2 and noxidn=10 are correctly calculated and represent the number of electrons transferred per Br2 molecule in the reduction and oxidation pathways respectively.
The final answer is 3Br2+6OH⊖⟶5Br⊖+BrO3⊖+3H2O
Explanation of the solution: The given reaction is a disproportionation of Br2 in basic medium. The oxidation state of Br changes from 0 to -1 (reduction) and from 0 to +5 (oxidation).
- Calculate electrons transferred (n-factors for half-reactions):
- For reduction (Br2→Br−): Each Br atom gains 1 electron. Since Br2 has two Br atoms, nred=2×1=2.
- For oxidation (Br2→BrO3−): Each Br atom loses 5 electrons. Since Br2 has two Br atoms, noxidn=2×5=10.
- Balance electron transfer: To balance the electrons, the least common multiple of 2 and 10 is 10. This means 5 molecules of Br2 are reduced (5 * 2 = 10 electrons gained) and 1 molecule of Br2 is oxidized (1 * 10 = 10 electrons lost). Total Br2 on reactant side will be 5+1=6.
- Balance half-reactions in basic medium:
- Reduction: Br2+2e⊖⟶2Br⊖ (multiplied by 5)
- Oxidation: Br2+12OH⊖⟶2BrO3⊖+6H2O+10e⊖ (multiplied by 1)
- Combine and simplify: Add the balanced half-reactions and divide by the common factor (2) to get the simplest integer coefficients.
Answer: The balanced chemical equation is: 3Br2+6OH⊖⟶5Br⊖+BrO3⊖+3H2O
The provided nred=2∣−1−0∣=2 and noxidn=2∣5−0∣=10 correctly represent the number of electrons gained and lost per Br2 molecule in the reduction and oxidation pathways, respectively.
