Question
Question: $\frac{1}{2\pi KL}$[lnx]$_a^b$=$\frac{1}{2\pi KL}$ ARJUNA A cylindrical brass boiler of radius 15c...
2πKL1[lnx]ab=2πKL1
ARJUNA
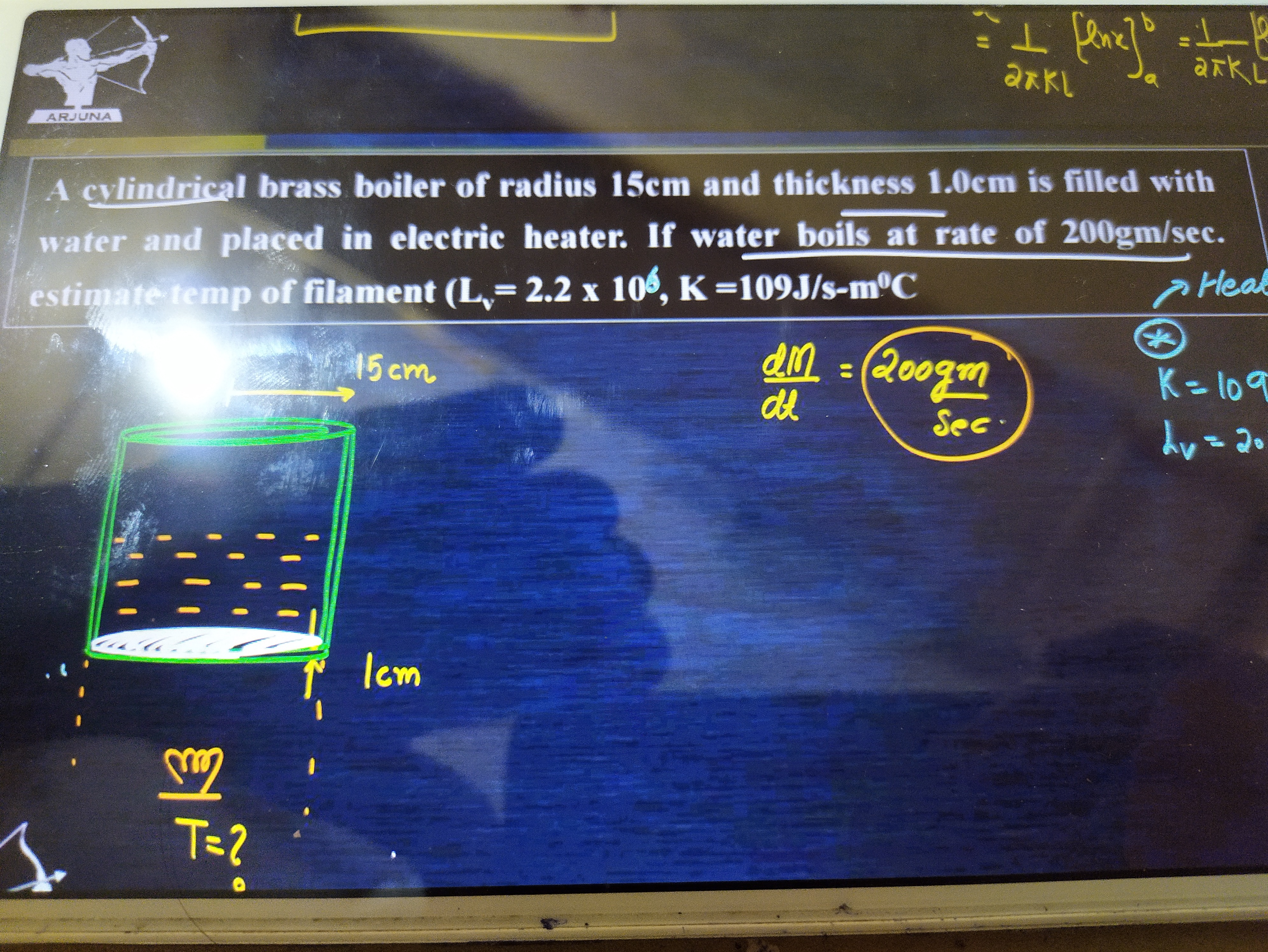
A cylindrical brass boiler of radius 15cm and thickness 1.0cm is filled with water and placed in electric heater. If water boils at rate of 200gm/sec. estimate temp of filament (Lv= 2.2 x 106, K=109J/s-m°C
671.07°C
Solution
The problem describes a cylindrical brass boiler with a given radius and thickness, which is used to boil water at a specified rate. We need to estimate the temperature of the electric heater's filament.
1. Identify Given Parameters:
- Radius of the boiler, r=15 cm=0.15 m
- Thickness of the boiler's base (through which heat is conducted), L=1.0 cm=0.01 m
- Rate of water boiling, dtdM=200 gm/sec=0.2 kg/sec
- Latent heat of vaporization of water, Lv=2.2×106 J/kg
- Thermal conductivity of brass, K=109 J/s-m∘C
- Boiling temperature of water (at atmospheric pressure), Twater=100∘C
2. Calculate the Area of Heat Transfer: Heat is transferred through the circular base of the cylindrical boiler. Area, A=πr2=π(0.15 m)2=0.0225π m2
3. Calculate the Rate of Heat Required to Boil Water: The rate at which heat is absorbed by the water to vaporize is given by: dtdQ=dtdM×Lv dtdQ=0.2 kg/s×2.2×106 J/kg=0.44×106 J/s=440000 W
4. Apply Fourier's Law of Heat Conduction: The rate of heat transfer by conduction through the boiler's base is given by: dtdQ=LKAΔT Where ΔT=Tfilament−Twater is the temperature difference across the thickness of the boiler.
5. Solve for the Filament Temperature (Tfilament): Equating the heat required for boiling to the heat transferred by conduction: 440000 W=0.01 m109 J/s-m∘C×(0.0225π m2)×(Tfilament−100∘C)
Rearranging the equation to solve for Tfilament: 440000×0.01=109×0.0225π×(Tfilament−100) 4400=(109×0.0225×π)×(Tfilament−100) 4400=(2.4525×π)×(Tfilament−100) Using π≈3.14159: 4400≈(2.4525×3.14159)×(Tfilament−100) 4400≈7.704665×(Tfilament−100)
Tfilament−100≈7.7046654400 Tfilament−100≈571.07∘C Tfilament≈571.07∘C+100∘C Tfilament≈671.07∘C
The temperature of the filament is approximately 671∘C.
Explanation of the solution:
- Calculate the heat transfer area (A=πr2).
- Determine the rate of heat required to vaporize water (dtdQ=dtdM×Lv).
- Apply Fourier's Law of heat conduction through the boiler's base (dtdQ=LKA(Tfilament−Twater)).
- Substitute known values and solve for Tfilament, assuming water boils at 100∘C.
Answer:
The estimated temperature of the filament is approximately 671.07∘C.
