Question
Question: Four containers are filled with monoatomic ideal gases. For each container, the number of moles, the...
Four containers are filled with monoatomic ideal gases. For each container, the number of moles, the mass of an individual atom and the rms speed of the atoms are expressed in terms of n, m and Vrms respectively. If TA, TB, TC and TD are their temperatures respectively then which one of the options correctly represents the order ?
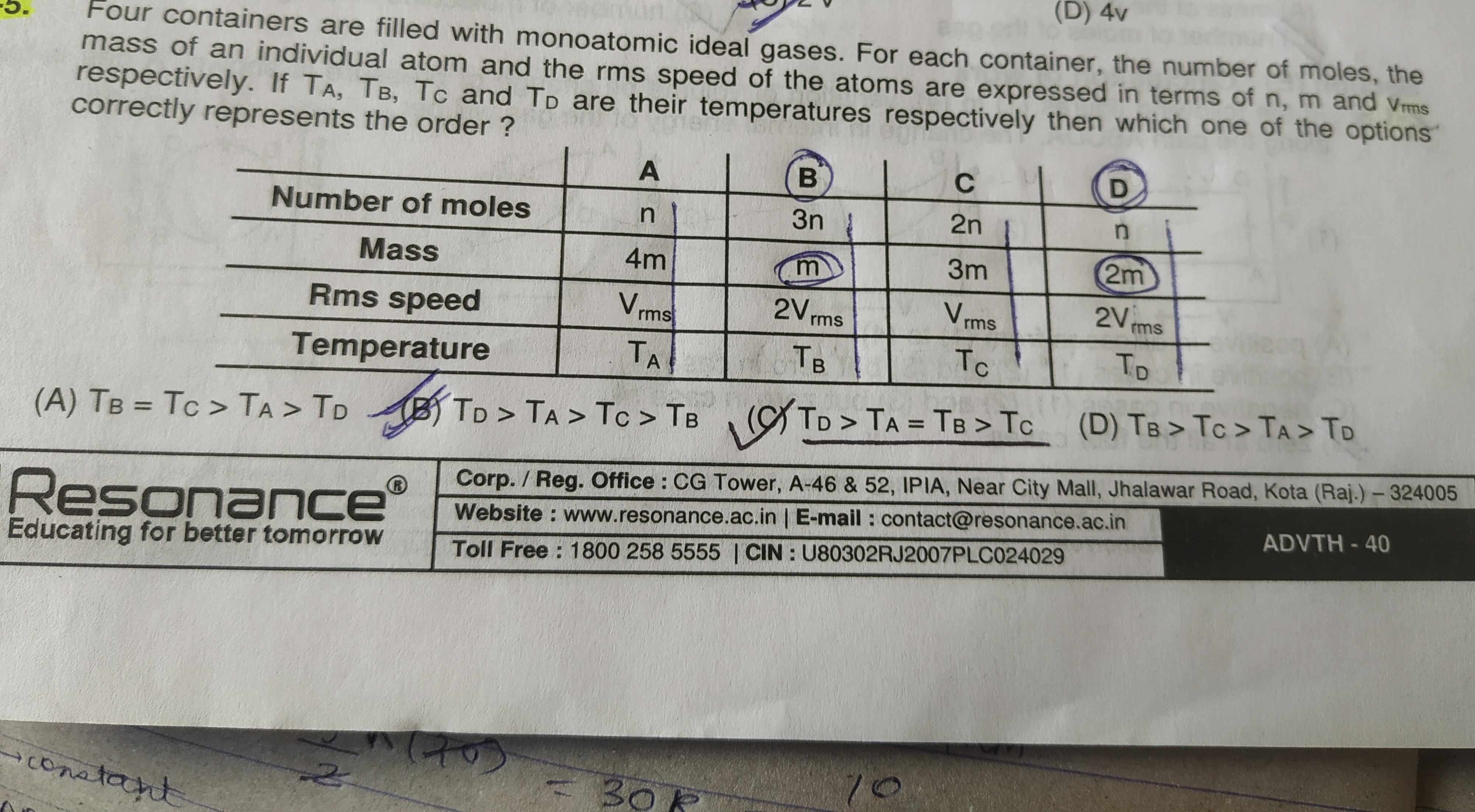
TB=TC>TA>TD
TD>TA>TC>TB
TD>TA=TB>TC
TB>TC>TA>TD
C
Solution
The root mean square (rms) speed of gas molecules is related to the absolute temperature (T) and the mass of an individual atom (m) by the formula:
Vrms=m3kT
where k is the Boltzmann constant.
Squaring both sides, we get:
Vrms2=m3kT
Rearranging the formula to express temperature (T):
T=3kmVrms2
Since 3k is a constant, the temperature T is directly proportional to the product of the mass of an individual atom (m) and the square of its rms speed (Vrms2). So, we can write T∝mVrms2.
Let's calculate the value of the product mVrms2 for each container (A, B, C, D) using the given data:
Container A: Mass of individual atom = 4m Rms speed = Vrms Temperature factor TA′=(4m)×(Vrms)2=4mVrms2
Container B: Mass of individual atom = m Rms speed = 2Vrms Temperature factor TB′=(m)×(2Vrms)2=m×(4Vrms2)=4mVrms2
Container C: Mass of individual atom = 3m Rms speed = Vrms Temperature factor TC′=(3m)×(Vrms)2=3mVrms2
Container D: Mass of individual atom = 2m Rms speed = 2Vrms Temperature factor TD′=(2m)×(2Vrms)2=2m×(4Vrms2)=8mVrms2
Now, let's compare these temperature factors: TA′=4mVrms2 TB′=4mVrms2 TC′=3mVrms2 TD′=8mVrms2
From the comparison, we can see the order of temperatures: TD>TA=TB>TC
This order matches option (C). The number of moles information is not required for this calculation.
