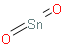Question
Question: Formula for the following compound Tin (IV) oxide is \(SnO_2\): (A) True (B) False...
Formula for the following compound Tin (IV) oxide is SnO2:
(A) True
(B) False
Solution
Hint: We should know that Tin dioxide is a tin oxide compound which consists of Tin (IV) covalently bound to two oxygen atoms. The chemical formula of Tin (IV) oxide, will further be understood from the structure of Tin (IV) oxide.
Complete step by step answer:
Tin (IV) oxide is also known as Stannic Oxide. We know that the symbol of Tin is Sn.
Here is the structure of Tin (IV) oxide, for better understanding of the formula:
The Tin (IV) crystallizes with the rutile structure. As such the Tin atoms are 6 coordinate and the oxygen atoms are 3 coordinate. SnO2 is usually regarded as an oxygen deficient n type semiconductor. Hydrous forms of SnO2 has been described as stannic acid.
The bond formation of Tin and the two oxygen atoms are known as just polar covalent. This is because of the electronegativity difference between Tin (1.8) and that of Oxygen (3.5). So, with a difference of only 1.7 in electronegativity, ionic bonds cannot be formed.
The chemical formula of Tin (IV) oxide is SnO2.
Therefore, the answer to the question that the formula for the following compound Tin (IV) oxide is True.
So Option A is the correct answer to the mentioned question.
- Tin (IV) oxide appears as white or off white crystalline solid or powder. It is insoluble in water and soluble in concentrated sulphuric acid and hydrochloric acid. It occurs in nature as a mineral cassiterite. It is used as a catalyst in putty as a polishing powder for steel and glass, in ceramic places and colours.
Note: As mentioned earlier SnO2 is insoluble in water. But it is also amphoteric in nature. By amphoteric we mean, it dissolves in acids and bases. It is also diamagnetic in nature.