Question
Question: Formula for the following compound Thallium (I) sulphate is \(T{l_2}S{O_4}\) . A.True B.False...
Formula for the following compound Thallium (I) sulphate is Tl2SO4 .
A.True
B.False
Solution
Thallium has 3 valence electrons (2 in 6s and 1 in 6p) and exhibits mostly (+1) oxidation state while sulphate (SO4−2) exhibits (-2) oxidation state.
Complete step by step answer:
-Thallium (Tl) has an atomic number of 81 and belongs to the Boron (B) group. It belongs to the 13th group and 6th period. Hence, its electronic configuration is: [Xe]4f145d106s26p1.
We can see from the configuration that Tl has the ability to lose 1 electron from the outermost subshell of 6p and attain a more stable state, thus exhibiting (+1) oxidation state. It can also lose 3 electrons from the outermost shell (6s and 6p). Thus thallium (Tl) can exhibit (+1) and (+3) oxidation states.
-Thallium (I) sulphate is also known as thallous sulphate is a sulphate salt of thallium in the common (+1) oxidation state as shown by Roman numeral (I).
-It is formed by the reaction of sulphuric acid with thallium metal, which is followed by crystallisation.
-Thallium sulphate is colourless, tasteless, odourless and highly toxic (hence used in insecticides, rodenticides and rat poisons. It gains its toxicity due to Tl+1 ion.
-Since the question says the name of the formula to be: Thallium (I) sulphate. The number (I) here shows the oxidation state of thallium atom, which is 1. And we all know that the sulphate has the molecular formula of SO4−2 and has an oxidation state of (-2). To satisfy or neutralise the (-2) charge of sulphate we will need a (+2) charge, but thallium has (+1) charge. So, we will require 2 atoms of thallium. Hence the molecular formula would be: Tl2SO4.
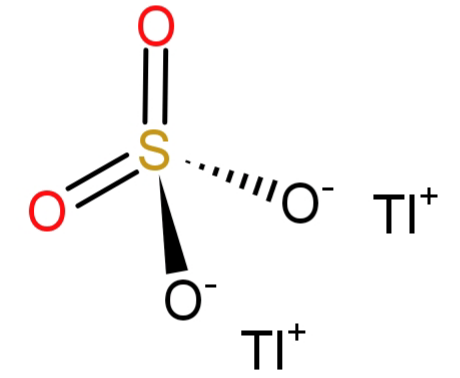
The statement is true and the correct option is: (A) True.
Note:
Although thallium can exhibit (+1) and (+3) oxidation states. It mostly prefers only (+1) because the 2 electrons from 6s subshell are relatively stable and much more difficult to remove than the 6p electron due to inert pair effect.
