Question
Question: Formula for ferrocene is A.\[{\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }...
Formula for ferrocene is
A.[Fe(CN)6]4 -
B.[Fe(CN)6]3 +
C.[Fe(CO)5]
D.[Fe(C5H5)2]
Solution
Ferrocene is actually a metallocene or we can say the first sandwich compound. Ferrocenes are the molecules where metal atoms are sandwiched between two parallel and planar cyclopentadienyl rings. Organometallic compound of Fe is ferrocene.
Complete step by step answer:
In ferrocene Fe metal is attached with a ligand called cyclopentadiene, the formula for cyclopentadiene is C5H5. In ferrocene, two cyclopentadienyl ligand present so the formula is [Fe(C5H5)2]. Two important conformers of ferrocene are: -
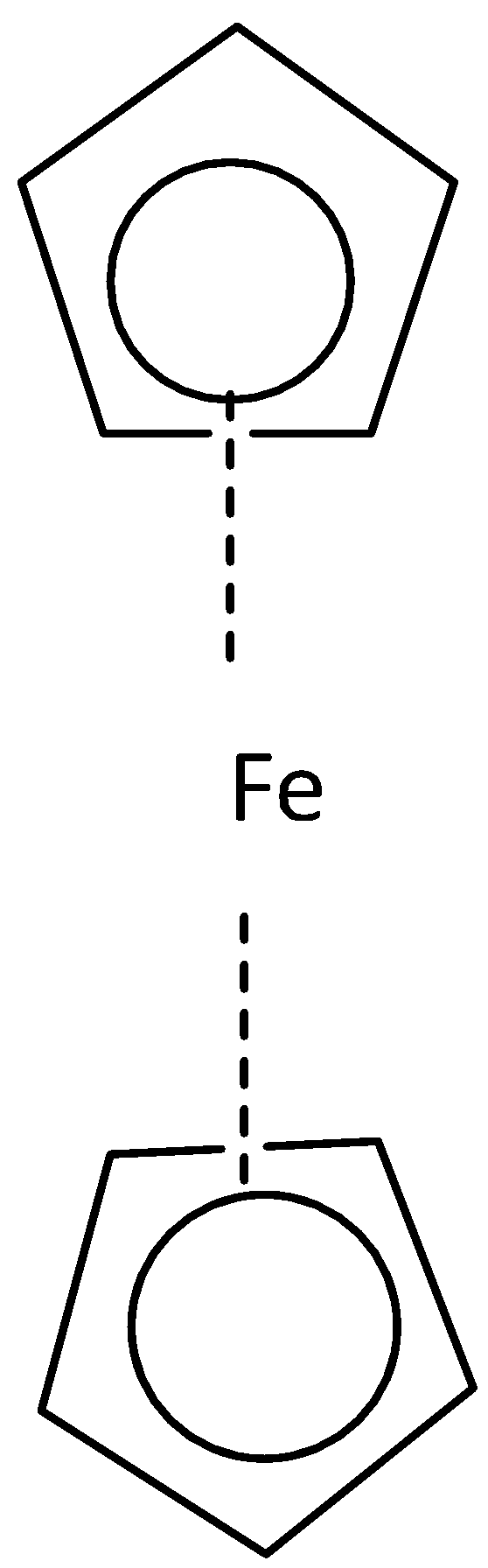
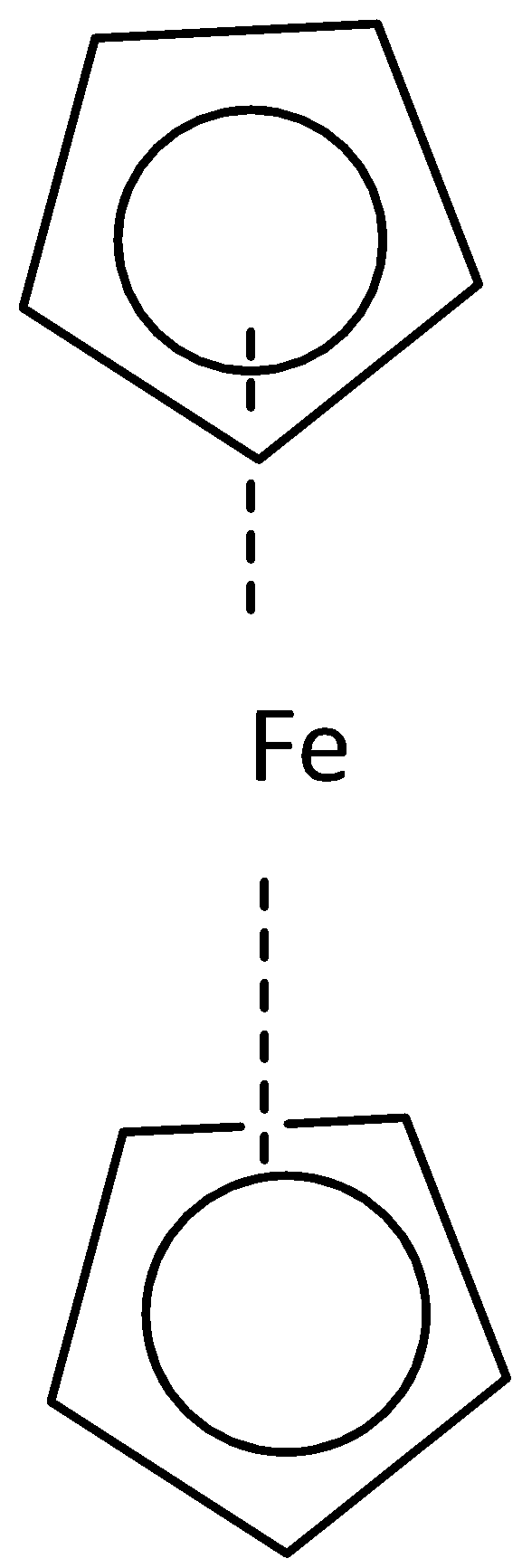
- (a) Staggered conformation (b) eclipsed conformation
Where both cyclopentadienyl rings are in the opposite direction that is the staggered conformation of ferrocene on the other hand where both the cyclopentadienyl rings in the same direction that is the eclipsed conformation. Point group for staggered conformation of ferrocene is D5d and for eclipsed conformation is D5h.
Therefore we can conclude that option D is the correct option, that is [Fe(C5H5)2]
Additional information:
Ferrocene undergoes electrophilic substitution reaction faster than benzene indicating that electrons of Cp ring are more readily available. Some electrophilic reactions of ferrocene are given ahead:
1.Friedel-Craft Acylation:
The reaction of ferrocene with acetyl chloride in the presence of AlCl3 produces mono acetyl derivative of ferrocene.
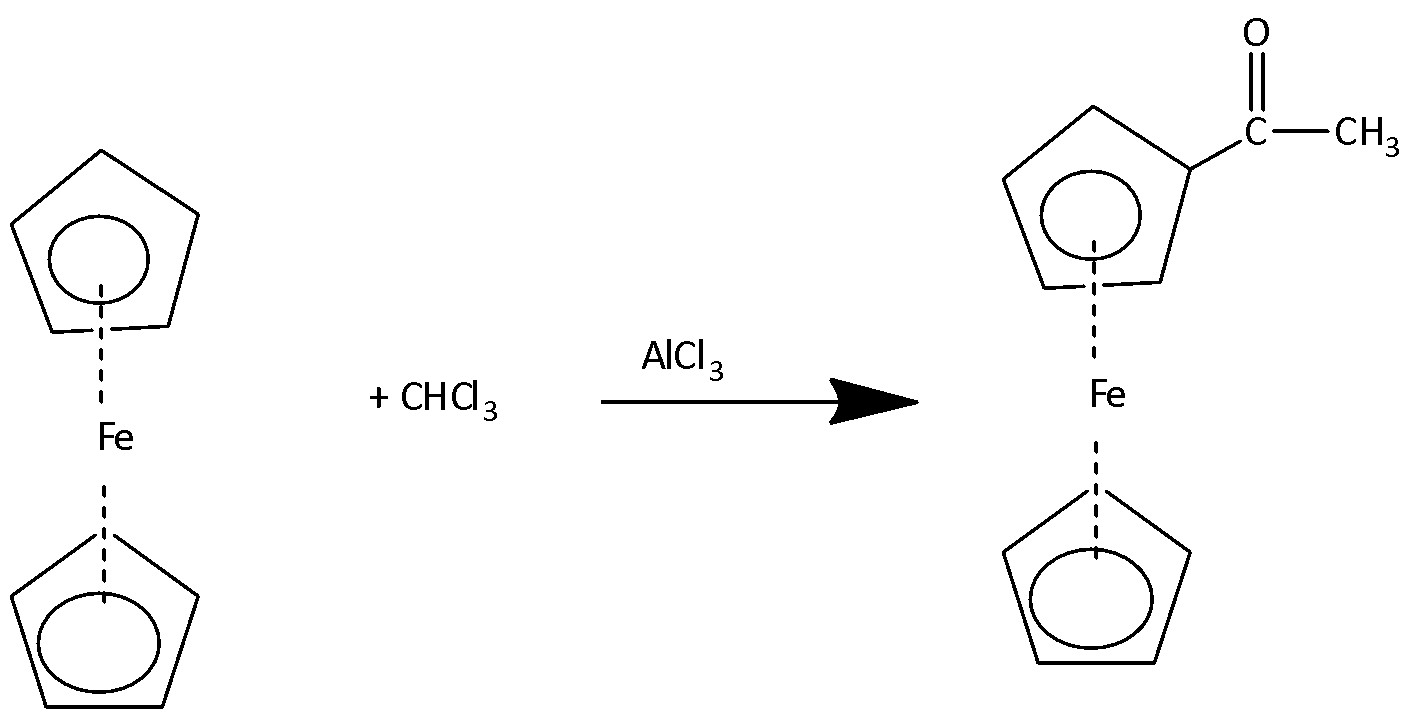
If the reaction is carried out in the presence of excess amounts of acetyl chloride then diacetyl derivatives of ferrocene are formed.
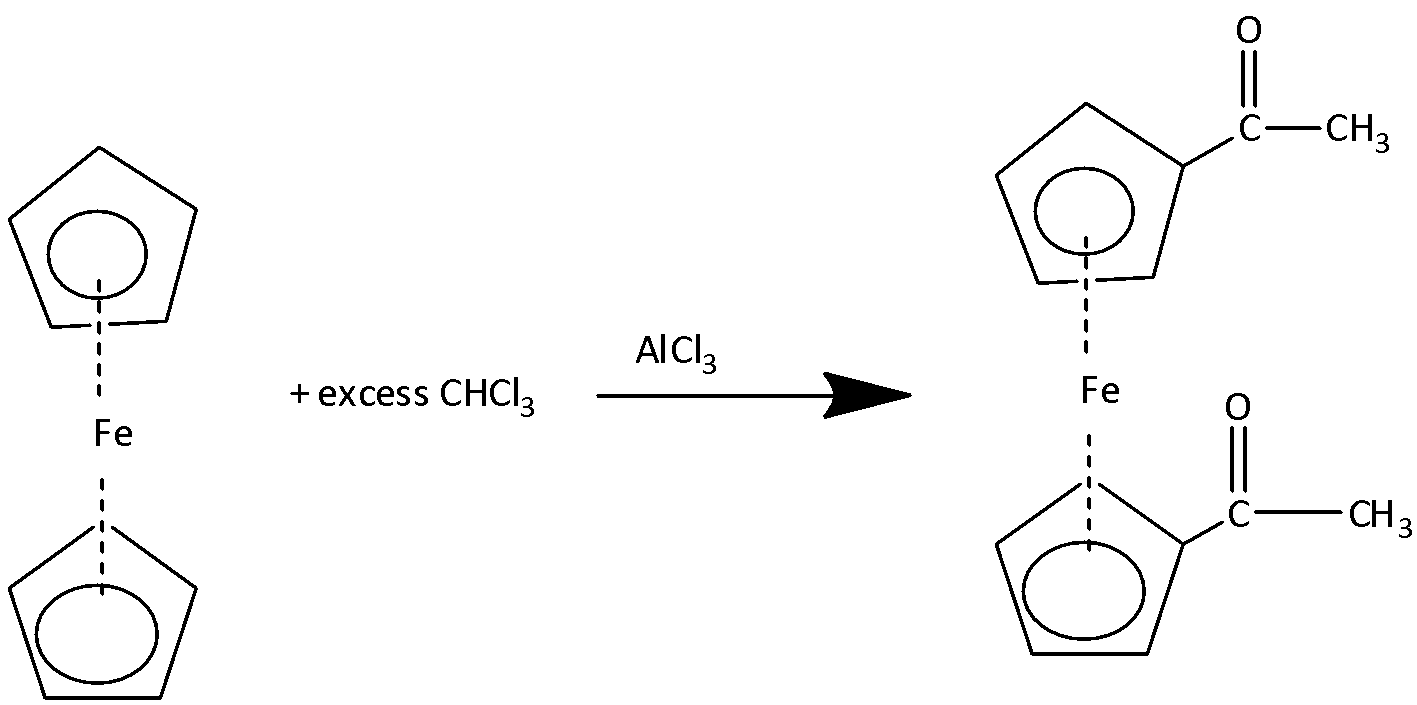
2,Friedel-Craft Alkylation:
In this reaction we used alkyl halide and AlCl3at 200 atmto produced mono alkyl derivative of ferrocene. If this reaction is carried out in the presence of excess amounts of alkyl chloride then the dialkyl derivative will be formed.
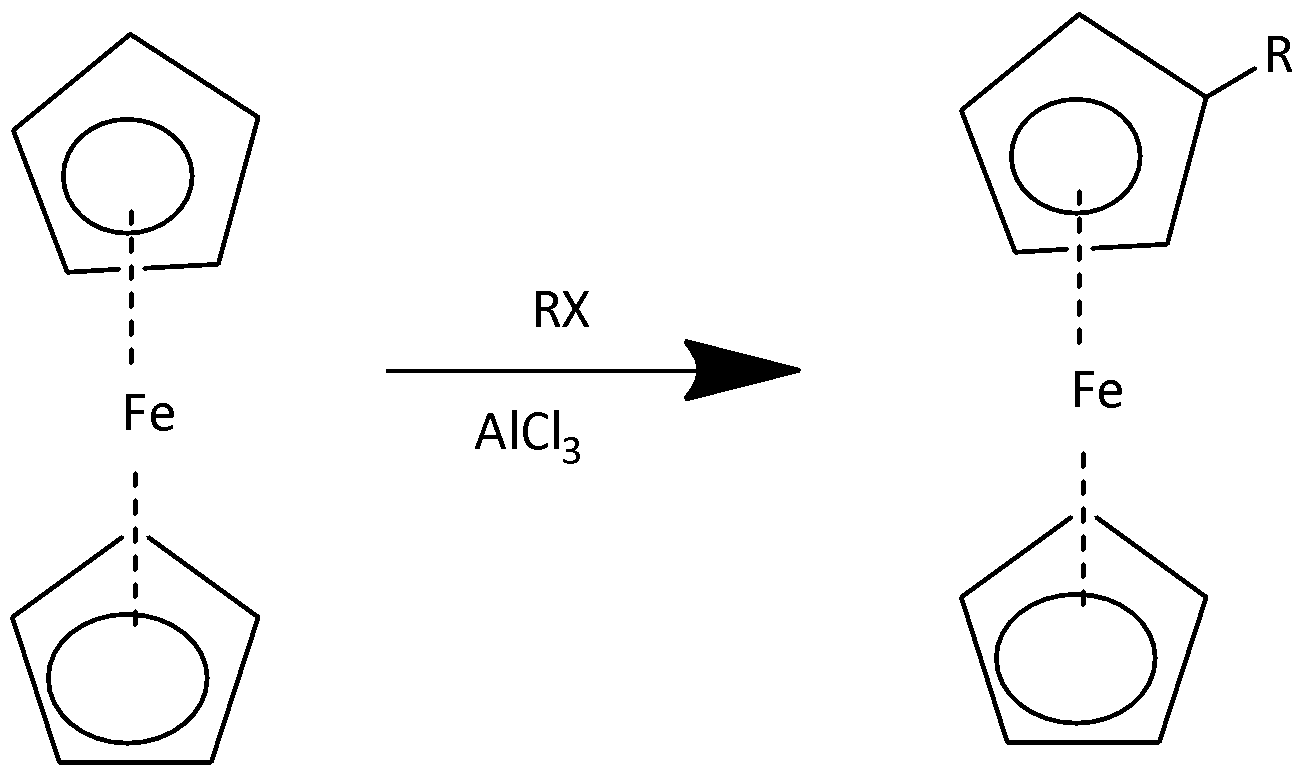
Note: The cyclopentadiene rings of metallocene are aromatic in nature therefore they do not undergo conjugate diene such as Diels alder reaction. They undergo electrophilic substitution reactions which are characteristic of aromatic compounds.
