Question
Question: For which of the given molecule dipole moments is not zero,\[\mu \ne 0\]? 
Solution
Dipole moment of the molecule defines the polarity of the bond between two atoms in a molecule. And this polarity between the bonds arises due to the development of the negative or positive charges on the atom sharing the bond.
Complete answer:
Now, we have the basic idea regarding the dipole moment and how it arises, so let’s look at each option.
One more point to keep in mind is that the direction of the dipole is from electronegative atom to the electropositive atom. Dipole moment is designated by the letterμ.
So looking at the figure (c)and(d) , quinoa and thioquinol molecules respectively. So the −OHgroup of the quinol molecule has different orientation as shown in figure, thus, it does not cancel out its dipole moment. So, its dipole momentμ=0.
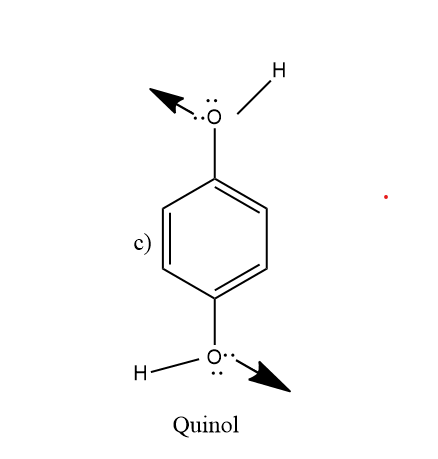
Similarly −SH group of thioquinol exists in different conformation and thus does not cancel out their dipole moment. So, its dipole moment is also not zero,μ=0.
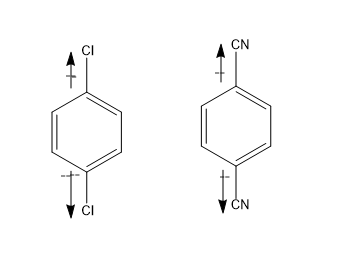
Whereas in case of figure (a)and(b) , chlorine being the electronegative atoms, at the both ends cancel out the dipole moment and thus its dipole moment is zero μ=0
Same is the case with cyanide groups, due to better symmetry it cancels out its dipole moment. μ=0.
So, option (b)is the correct answer i.e. figure (c)and(d) .
Note:
While attempting this type of question, make sure to read the question very carefully, because they are asking for the figure which “do not “show the dipole moment zero. So keep in mind the word not because usually in a hurry we neglect.
