Question
Question: For the solution of the gases w, x, y, and z in the water at \(\text{ 298 K }\) , Henry’s law consta...
For the solution of the gases w, x, y, and z in the water at 298 K , Henry’s law constant (kH) is 0.5 , 2.0 , 35 and 40 kbar , respectively. The correct plot for the given data is :
(A) 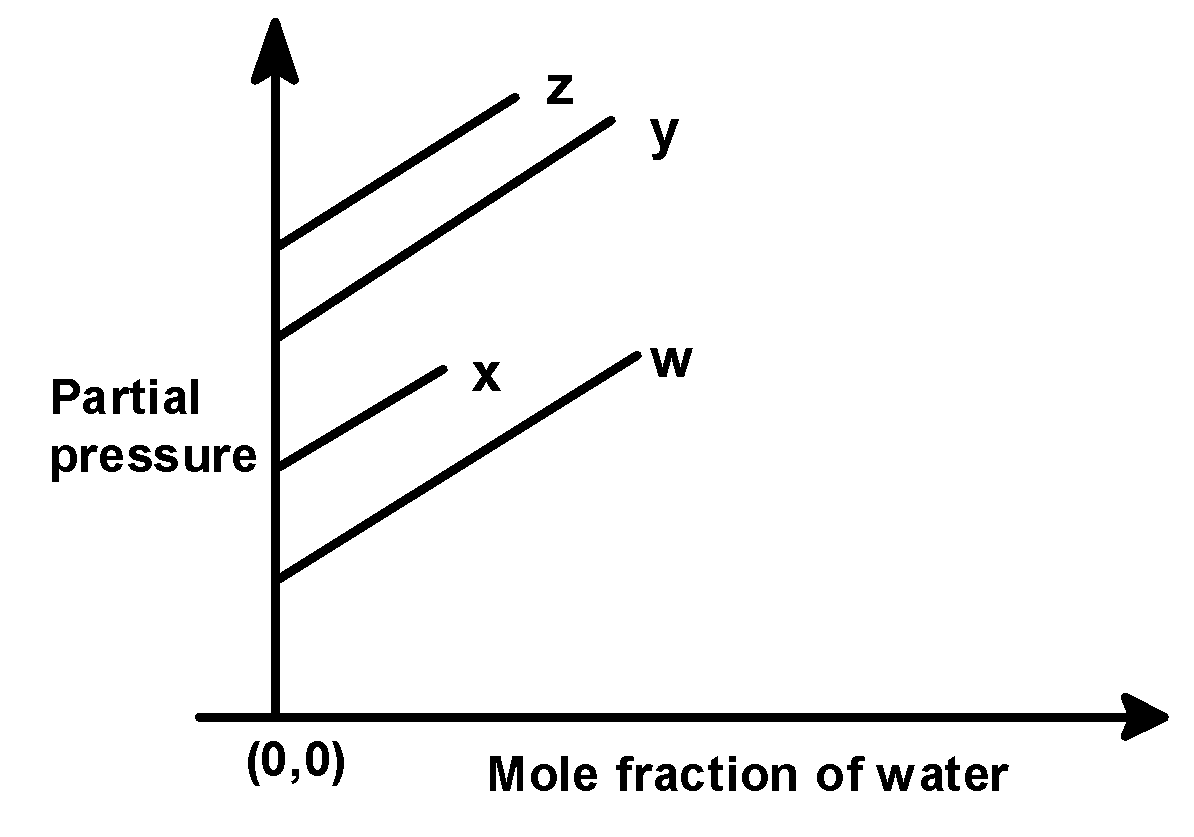
(B) 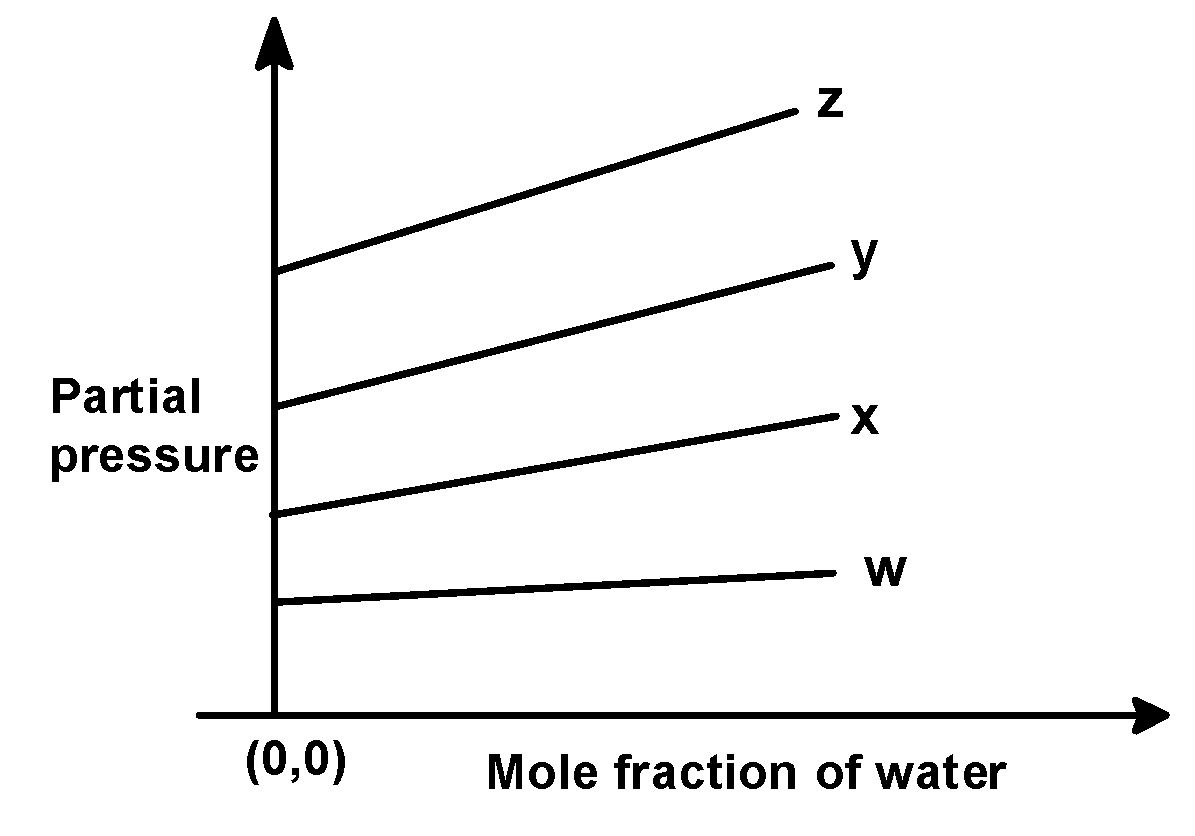
(C) 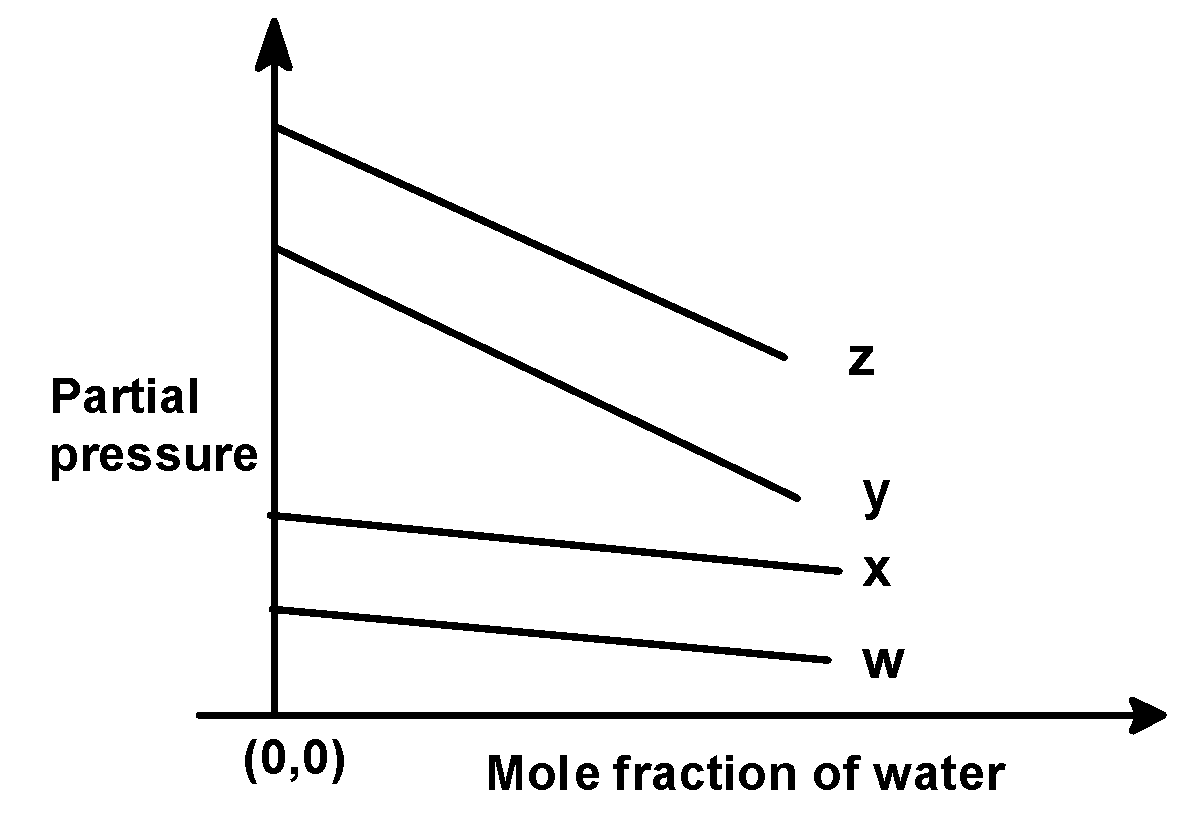
(D) 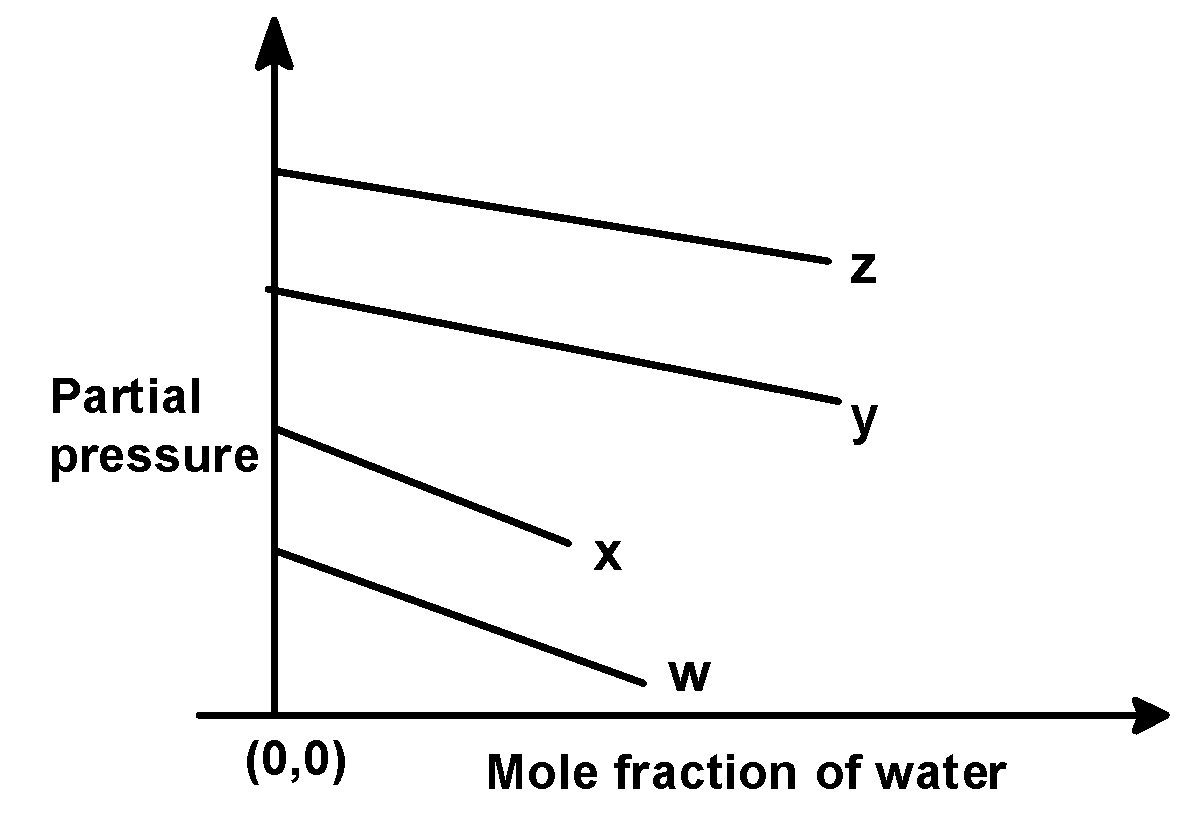
Solution
The pressure has a remarkable effect on the solubility of the gas. The relation between the partial pressure and mole fraction of a gas solute is given as,
pgas = kH × χgas (1)
Where χgas the mole fraction of solute and kH is henry’s constant. The plot of the mole fraction of gas against the partial pressure is a straight line and follows the straight-line equation y = mx + C .
Complete step by step answer:
-The solubility of gases at a given temperature increases directly as the pressure. This conclusion forms a basis of what is known as Henry’s law, which may be stated as below.
-The mass of a gas dissolved per unit volume of a solvent is proportional to the pressure of the gas in equilibrium with the solution at a constant temperature.
-The henry law can be alternatively stated as the pressure of the gas over the solution in which the gas is dissolved is proportional to the mole fraction of the gas dissolved in solution. That is,
pgas = kH × χgas (1)
Where χgas the mole fraction of solute and kH is henry’s constant.
-If gas is dissolved in the water then the sum of the mole fraction of the water as gas would be equal to 1. That is,
χH2O + χgas = 1or χgas = 1−χH2O
Thus, equation (1) would become,
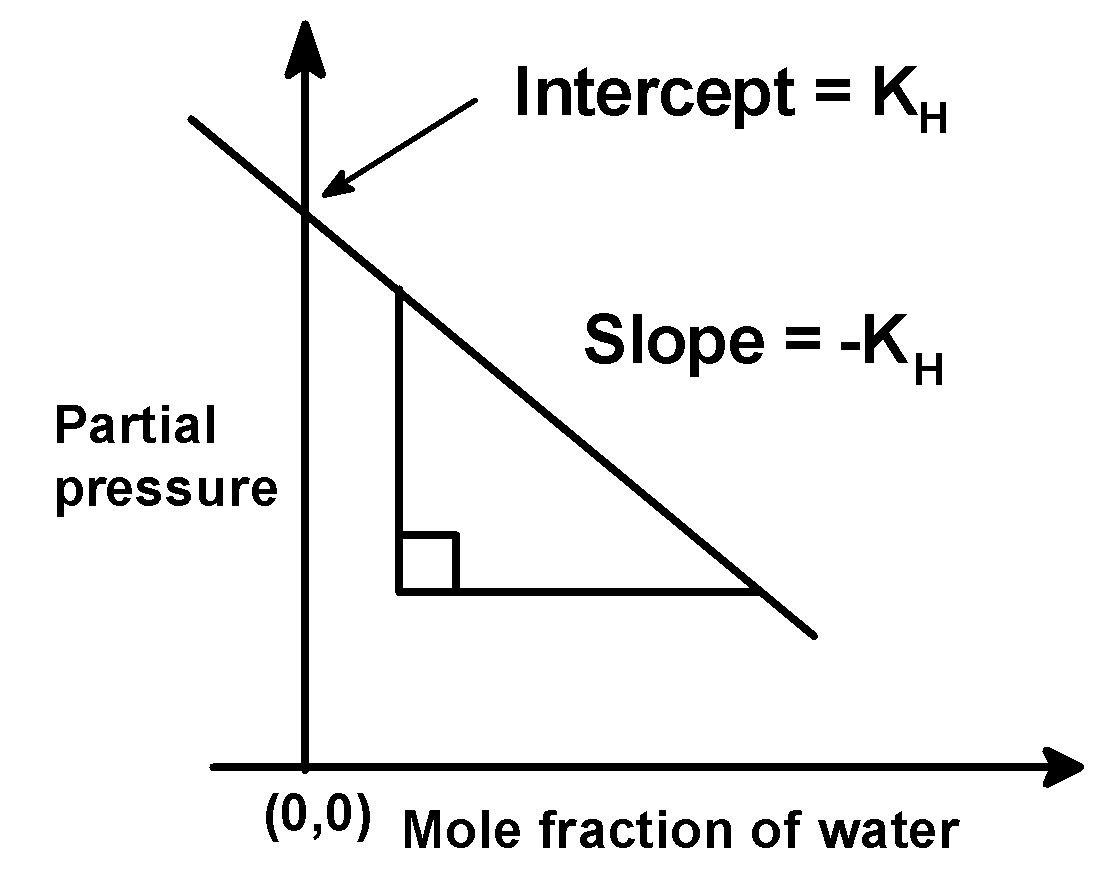
pgas = kH × (1−χH2O)⇒pgas = kH−kH×χH2O⇒pgas = (−kH)×χH2O + kH
The equation pgas = (−kH)×χH2O + kH (2)
Seems similar to the equation of the straight line which is,
y = mx + C (3)
Where m is the slope and C is the intercept.
On comparing (2) and (3) we get that,
The slope is (−kH) an intercept is (kH) .
In the problem, we have given the following intercepts,
w = 0.5 , x = 2.0 , y = 35 and z = 40 kilo bar. Thus the increasing order of intercepts for the given plot would be written as,
w < x < y < z
This order is seen in plot C).
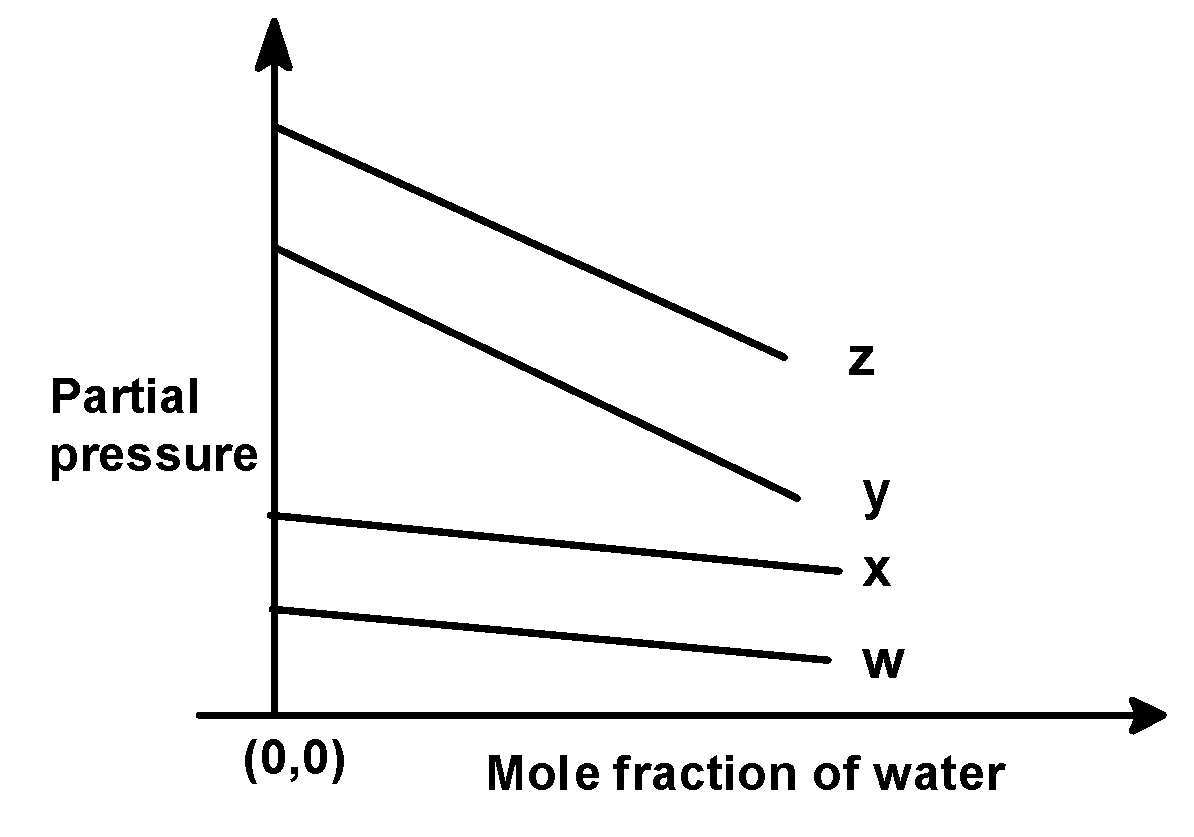
Hence, (C) is the correct option.
Note: Note that, if a gas obeys a henry's law then the graph obtained by plotting solubility of gas (i.e. Mole fraction of solute) against the pressure at the constant pressure would be a straight line.
The gas obeys the henry's law provided,
1. Pressure is not too high
2. Temperature is not too low
3. Gas is not highly soluble and does not dissociate or associate in the solution.
