Question
Question: For the reactions (I) cyclohexyl–Cl → cyclohexyl+ + Cl−, ΔH1o (II) cyclohexenyl–Cl → cyclohexenyl...
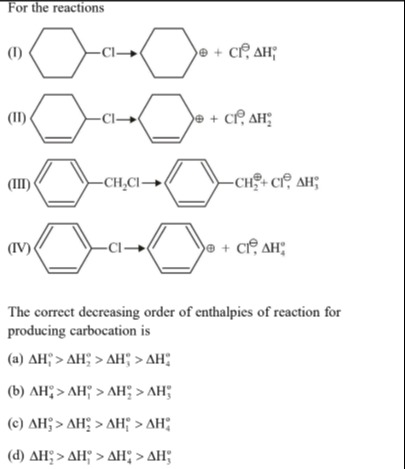
For the reactions
(I) cyclohexyl–Cl → cyclohexyl+ + Cl−, ΔH1o
(II) cyclohexenyl–Cl → cyclohexenyl+ + Cl−, ΔH2o
(III) benzyl–CH2Cl → benzyl+ + Cl−, ΔH3o
(IV) phenyl–Cl → phenyl+ + Cl−, ΔH4o
The correct decreasing order of enthalpies of reaction for producing carbocation is
A
ΔH1o > ΔH2o > ΔH3o > ΔH4o
B
ΔH4o > ΔH1o > ΔH2o > ΔH3o
C
ΔH3o > ΔH2o > ΔH1o > ΔH4o
D
ΔH2o > ΔH1o > ΔH4o > ΔH3o
Answer
ΔH2o > ΔH1o > ΔH4o > ΔH3o
Explanation
Solution
Key concept: Lower stability of the carbocation → higher ΔH° of its formation.
- (II) cyclohexenyl+ is a vinylic cation and is very unstable → largest ΔH2o.
- (I) cyclohexyl+ is a secondary alkyl cation → moderately unstable → next highest ΔH1o.
- (IV) phenyl+ is an aryl cation (no resonance stabilization) → more unstable than benzyl but more stable than vinylic → ΔH4o follows.
- (III) benzyl+ is highly resonance–stabilized → lowest ΔH3o.
Therefore the decreasing order is
ΔH2o>ΔH1o>ΔH4o>ΔH3o
