Question
Question: For the reaction, X(s) $\rightleftharpoons$ Y(s) + Z (g), the plot of $ln \frac{p_z}{p^{\theta}}$ ve...
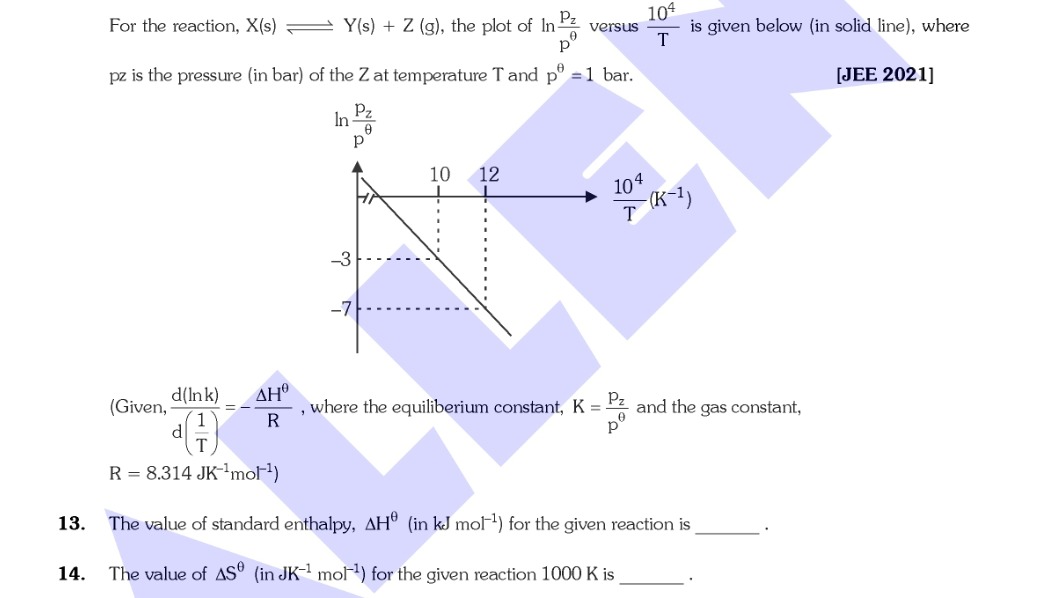
For the reaction, X(s) ⇌ Y(s) + Z (g), the plot of lnpθpz versus T104 is given below (in solid line), where pz is the pressure (in bar) of the Z at temperature T and pθ=1 bar.
[JEE 2021]
(Given, d(T1)d(lnk)=−RΔHθ, where the equiliberium constant, K=pθpz and the gas constant, R=8.314 JK−1mol−1)
Answer
- 166.28 kJ mol−114. 141.34 JK−1 mol−1
Explanation
Solution
-
The slope of the plot of lnK vs T104 is m=−104RΔHθ. From the graph, m=−2. Thus, ΔHθ=−m⋅104⋅R=−(−2)⋅104⋅8.314=166280 J/mol =166.28 kJ/mol.
-
The y-intercept of the plot of lnK vs T104 corresponds to RΔSθ. The line equation is y=−2x+17, so the intercept is 17. Thus, RΔSθ=17, which gives ΔSθ=17⋅R=17⋅8.314=141.338 JK−1mol−1.
