Question
Chemistry Question on Law Of Chemical Equilibrium And Equilibrium Constant
For the reaction, X(s)⇌Y(s)+Z(g), the plot of lnp0pZ versus T104 is given below (in solid line), where pz is the pressure (in bar) of the gas Z at temperature T and p0=1 bar
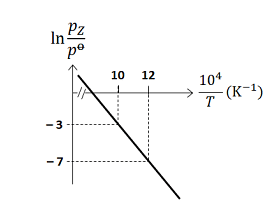
(Given, d(T1)d(lnK)=−RΔH0, where the equilibrium constant, K=p0pz and the gas constant, R=8.314JK−1mol−1 )
The value of ΔS0 (in JK−1mol−1 ) for the given reaction, at 1000K is ______
Answer
The value of ΔS0 (in JK−1mol−1 ) for the given reaction, at 1000K is 141.34.
Explanation
Solution
The value of ΔS0 (in JK−1mol−1 ) for the given reaction, at 1000K is 141.34.
