Question
Question: For the reaction $N_2(g) + O_2(g) \rightleftharpoons 2NO(g)$, the value of $K_c$ at $800^\circ C$ is...
For the reaction N2(g)+O2(g)⇌2NO(g), the value of Kc at 800∘C is 0.1. When the equilibrium concentration of both the reactants is 0.5 mol, the value of Kp at this temperature is x×10−3. Then what is x?
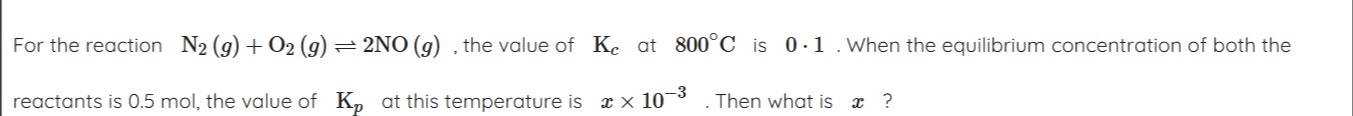
A
100
B
10
C
1
D
0.1
Answer
100
Explanation
Solution
For the reaction N2(g)+O2(g)⇌2NO(g), the change in the number of moles of gas is Δn=(moles of gaseous products)−(moles of gaseous reactants)=2−(1+1)=0. The relationship between Kp and Kc is given by Kp=Kc(RT)Δn. Since Δn=0, the equation simplifies to Kp=Kc. Given Kc=0.1, therefore Kp=0.1. The problem states that Kp=x×10−3. So, 0.1=x×10−3. Solving for x: x=10−30.1=0.1×103=100. The equilibrium concentration of reactants is irrelevant when Δn=0.
