Question
Question: For the reaction: \({{N}_{2}}{{O}_{5}}(g)\to {{N}_{2}}{{O}_{4}}(g)+\dfrac{1}{2}{{O}_{2}}(g)\), the i...
For the reaction: N2O5(g)→N2O4(g)+21O2(g), the initial pressure is 114mm and after 20 seconds, the pressure of the reaction mixture becomes 133mm. Then the average rate of reaction will be:
A. 1.9 atm S−1
B. 8.75×10−3 atm S−1
C. 2.5×10−3 atm S−1
D. 6.65 atm S−1
Solution
You can apply the simple formula for finding the average rate of the reaction by dividing the change in pressure and change in time in the reaction. Already the initial pressure, total pressure and change in time is given. So, now you can find the change in pressure from the reaction given.
Complete step by step solution:
Given that,
The reaction involved is:
N2O5(g)→N2O4(g)+21O2(g)
The initial pressure for the reaction at time 0 second is given as 114mm.
The total pressure of the reaction mixture after 20 seconds is given as 133mm.
So, the change in time of the reaction is (20−0)=20 seconds.
Let’s consider the change in pressure as α.
The initial pressure (at zero second) of N2O5, N2O4 and O2 will be 114mm, 0mm and 0mm.
The pressure at 20 seconds for N2O5, N2O4 and O2 will be 114−α, α and 2α.
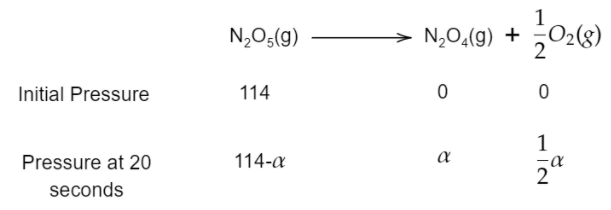
Therefore, the total pressure will be 114−α+α+2α=133mm.
Then, 114+2α=133mm
Thus, 2α=(133−114)mm=19mm
So, α=19×2=38mm
We know that one atmosphere equals to 760mm.
So, converting 38mm to the atmosphere will be 76038=0.05atm.
Thus, the change in pressure will be 0.05atm.
The average rate of the reaction can be calculated by using the formula of dividing the change in pressure to that of the change in time.
So, the average rate of the reaction will be
rate=change in timechange in pressure=20s0.05atm=2.5×10−3atm s−1
Hence, the correct option is C.
Note: While calculating keep in mind about the units. The rate of a chemical reaction determines the speed of the reaction at which it tends to proceed. It is generally expressed in terms of either the concentration of a product formed in a unit time or the concentration of a reactant consumed in a unit time.
