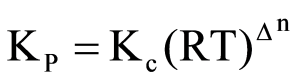Question
Question: For the reaction <img src="https://cdn.pureessence.tech/canvas_511.png?top_left_x=300&top_left_y=136...
For the reaction 
 N2O4( g),KP/Kc is equal to.
N2O4( g),KP/Kc is equal to.
A
RT1
B
RT
C
RT
D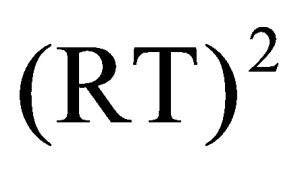
Answer
RT1
Explanation
Solution
: 
 N2O4( g)
N2O4( g)
Δn=1−2=−1