Question
Question: Using data given in table find out incorrect statement(s) among the following. $CO(g) + \frac{1}{2}...
Using data given in table find out incorrect statement(s) among the following.
CO(g)+21O2(g)→CO2(g)
Assume vibration modes of motion do not contribute to heat capacity at low temperature.
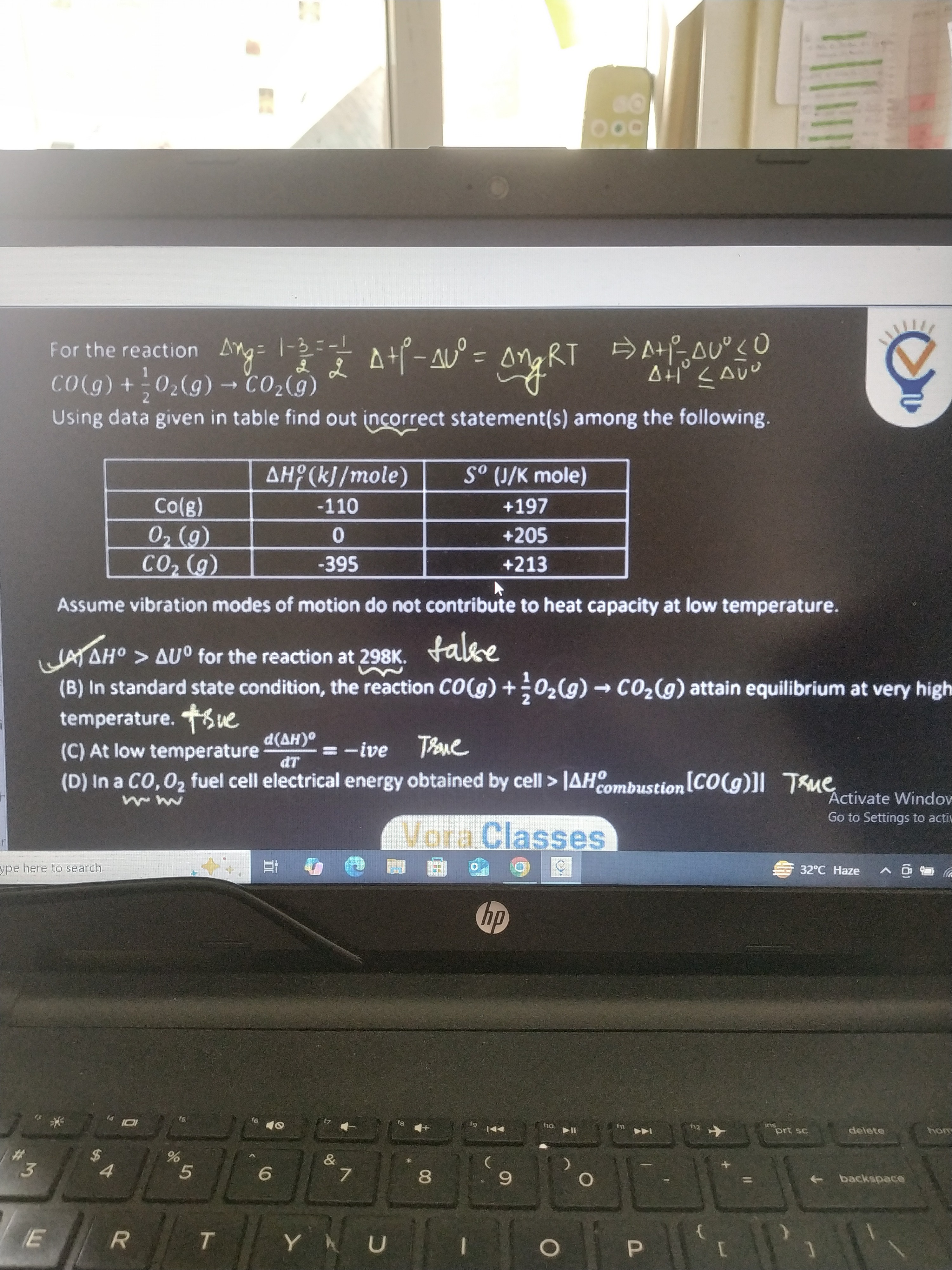
ΔH∘>ΔU∘ for the reaction at 298K.
In standard state condition, the reaction CO(g)+21O2(g)→CO2(g) attain equilibrium at very high temperature.
At low temperature dTd(ΔH)∘=−ive
In a CO,O2 fuel cell electrical energy obtained by cell >∣ΔHcombustion∘[CO(g)]∣
A, D
Solution
The reaction is CO(g)+21O2(g)→CO2(g).
(A) ΔH∘>ΔU∘ for the reaction at 298K. The relationship between ΔH∘ and ΔU∘ is ΔH∘=ΔU∘+ΔngRT, where Δng is the change in the number of moles of gas. For the given reaction, Δng=moles of gaseous products−moles of gaseous reactants=1−(1+21)=1−23=−21. At 298K, R and T are positive. Thus, ΔngRT=−21RT is negative. ΔH∘−ΔU∘=ΔngRT<0, which means ΔH∘<ΔU∘. Therefore, the statement ΔH∘>ΔU∘ is incorrect.
(B) In standard state condition, the reaction CO(g)+21O2(g)→CO2(g) attain equilibrium at very high temperature. For equilibrium, ΔG∘=0. ΔG∘=ΔH∘−TΔS∘. The equilibrium temperature is Teq=ΔS∘ΔH∘. Using the given data: ΔH∘=ΔHf∘(CO2)−[ΔHf∘(CO)+21ΔHf∘(O2)]=−395−[−110+21(0)]=−395+110=−285kJ/mol. ΔS∘=S∘(CO2)−[S∘(CO)+21S∘(O2)]=213−[197+21(205)]=213−[197+102.5]=213−299.5=−86.5J/K mol=−0.0865kJ/K mol. Teq=−0.0865kJ/K mol−285kJ/mol≈3294.8K. Since the equilibrium temperature is very high, the statement is true.
(C) At low temperature dTd(ΔH)∘=−ive. According to Kirchhoff's law, dTd(ΔH)∘=ΔCp∘. At low temperature, assuming only translational and rotational contributions to heat capacity for ideal gases: For linear molecules (CO, O2, CO2), Cp=CV+R=(23R+R)+R=25R+R=27R (translational + rotational + R). Or, using degrees of freedom: CV=2fR, Cp=CV+R. Translational degrees of freedom = 3 for all gases. Rotational degrees of freedom = 2 for linear molecules. CV(linear)=23R+22R=25R. Cp(linear)=25R+R=27R. ΔCp∘=Cp(CO2)−[Cp(CO)+21Cp(O2)]=27R−[27R+21(27R)]=27R−27R−47R=−47R. Since R is positive, ΔCp∘ is negative. So, dTd(ΔH)∘=ΔCp∘ is negative. The statement is true.
(D) In a CO,O2 fuel cell electrical energy obtained by cell >∣ΔHcombustion∘[CO(g)]∣. The maximum electrical work obtained from a fuel cell operating reversibly at constant temperature and pressure is given by −ΔG∘. The enthalpy of combustion of CO is ΔHcombustion∘[CO(g)]=ΔH∘=−285kJ/mol. ∣ΔHcombustion∘[CO(g)]∣=∣−285∣=285kJ/mol. The electrical energy obtained is −ΔG∘=−(ΔH∘−TΔS∘)=−ΔH∘+TΔS∘. We need to check if −ΔH∘+TΔS∘>−ΔH∘. This inequality simplifies to TΔS∘>0. We calculated ΔS∘=−86.5J/K mol. At any positive temperature T, TΔS∘ will be negative. Since TΔS∘<0, it means −ΔH∘+TΔS∘<−ΔH∘. So, the electrical energy obtained is less than the magnitude of the enthalpy of combustion. The statement is incorrect.
The incorrect statements are (A) and (D).
