Question
Question: For the reaction A(g) + 2B(g) $\rightleftharpoons$ C(g) + D(g) ; K$_c$ = 10$^{12}$. If the initial ...
For the reaction A(g) + 2B(g) ⇌ C(g) + D(g) ; Kc = 1012.
If the initial moles of A,B,C and D are 0.5, 1, 0.5 and 3.5 moles respectively in a one litre vessel. What is the equilibrium concentration of B (in millimole/litre) ?
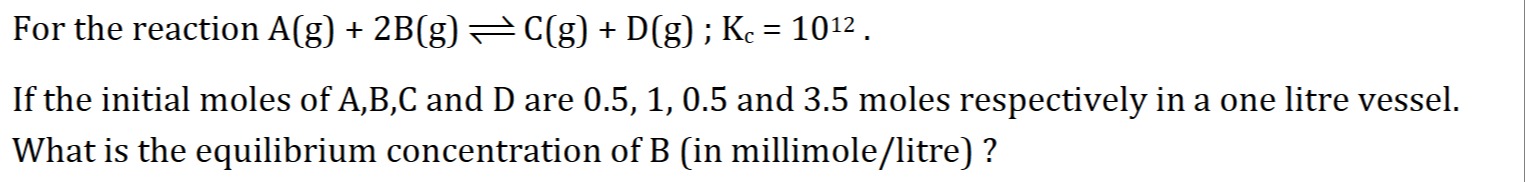
0.2
Solution
The reaction is A(g) + 2B(g) ⇌ C(g) + D(g). The equilibrium constant Kc = 1012. The initial moles are: A = 0.5 mol, B = 1 mol, C = 0.5 mol, D = 3.5 mol. The volume of the vessel is 1 L. Therefore, initial concentrations are equal to initial moles.
-
Calculate the reaction quotient (Qc): Qc=[A]0[B]02[C]0[D]0=(0.5)(1)2(0.5)(3.5)=0.51.75=3.5
-
Determine the direction of the reaction: Since Qc (3.5) < Kc (1012), the reaction will proceed in the forward direction to reach equilibrium.
-
Analyze the extent of reaction due to large Kc: A very large Kc value (1012) indicates that the reaction proceeds almost to completion in the forward direction. Let's determine the limiting reactant for the forward reaction: Initial moles of A = 0.5 mol Initial moles of B = 1 mol According to the stoichiometry (1 mole of A reacts with 2 moles of B): If 0.5 mol of A reacts, it consumes 0.5 * 2 = 1 mol of B. Since we have exactly 0.5 mol of A and 1 mol of B, both reactants A and B will be almost entirely consumed if the reaction goes to completion.
-
Set up an ICE table by assuming complete reaction first, then a small back reaction: Assume the reaction goes to completion in the forward direction: A(g) + 2B(g) ⇌ C(g) + D(g) Initial (mol): 0.5 1 0.5 3.5 Change (mol): -0.5 -1 +0.5 +0.5 After complete reaction (mol): 0 0 1 4
Now, since equilibrium must be established (meaning all species are present, even if in very small amounts), a very small amount of products will react backward. Let 'y' be the moles of C that react in the reverse direction to establish equilibrium. C(g) + D(g) ⇌ A(g) + 2B(g) Initial (after forward completion, mol): 1 4 0 0 Change (mol): -y -y +y +2y Equilibrium (mol): (1-y) (4-y) y 2y
Since the volume of the vessel is 1 L, these moles are also the equilibrium concentrations in mol/L.
-
Substitute equilibrium concentrations into the Kc expression: Kc=[A][B]2[C][D] 1012=(y)(2y)2(1−y)(4−y) 1012=4y3(1−y)(4−y)
-
Solve for 'y' using approximation: Since Kc is very large, 'y' is expected to be very small. Therefore, we can approximate: (1-y) ≈ 1 (4-y) ≈ 4 Substituting these approximations: 1012≈4y3(1)(4) 1012≈4y34 1012≈y31 y3≈10121=10−12 y≈(10−12)1/3 y≈10−4 mol/L
-
Calculate the equilibrium concentration of B: From the equilibrium concentrations in the ICE table, [B]eq = 2y. [B]eq = 2 * 10−4 mol/L
-
Convert the concentration to millimole/litre: 1 mole = 1000 millimoles [B]eq = 2 * 10−4 mol/L * (1000 mmol/mol) [B]eq = 2 * 10−4 * 103 mmol/L [B]eq = 2 * 10−1 mmol/L [B]eq = 0.2 mmol/L
The final answer is 0.2.
Explanation of the solution:
- Calculate initial reaction quotient Qc.
- Compare Qc with Kc to determine reaction direction. Qc < Kc, so reaction proceeds forward.
- Since Kc is very large, the reaction goes almost to completion. Determine limiting reactants (A and B are both limiting).
- Assume reaction goes to completion, then a small amount 'y' of products react backward to establish equilibrium.
- Set up equilibrium concentrations in terms of 'y'.
- Substitute into Kc expression and use approximation (1-y ≈ 1, 4-y ≈ 4) due to small 'y'.
- Solve for 'y'.
- Calculate [B] = 2y.
- Convert mol/L to millimole/L.
