Question
Question: For the reaction A → B the following graph was obtained. The time required (in seconds) for the conc...
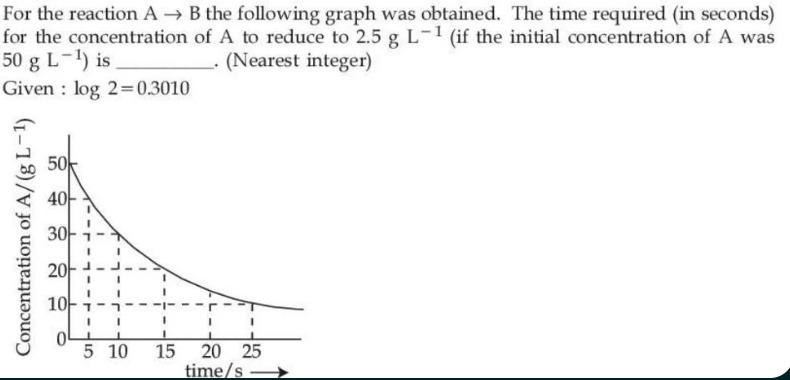
For the reaction A → B the following graph was obtained. The time required (in seconds) for the concentration of A to reduce to 2.5 g L−1 (if the initial concentration of A was 50 g L−1) is ________. (Nearest integer) Given : log 2 = 0.3010
Answer
28 s
Explanation
Solution
For a first‐order reaction, the integrated rate law is
ln[A][A]0=kt.Given that at t=15s, [A]=10g/L with [A]0=50g/L, we compute the rate constant k:
ln1050=ln5=k×15⟹k=15ln5.To find the time when [A]=2.5g/L:
ln2.550=ln20=kt.Substitute k:
t=ln5/15ln20=ln515ln20.Using the relation ln20=ln5+ln4 (and approximate values ln5≈1.609 and ln4≈1.386):
t≈15×1.6091.609+1.386≈15×1.861≈27.91s.Rounding to the nearest integer gives 28 s.
