Question
Question: For the reaction: $2Fe^{3+}$ aq + $2I^-$aq $\rightarrow$ $2Fe^{2+}$ aq + $I_2s$ The magnitude of th...
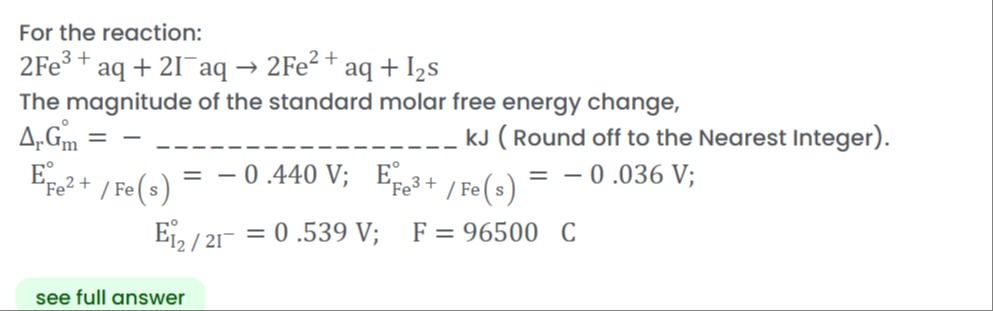
For the reaction: 2Fe3+ aq + 2I−aq → 2Fe2+ aq + I2s
The magnitude of the standard molar free energy change, ΔrGmo = - ______________ kJ ( Round off to the Nearest Integer).
A
45
B
44
C
46
D
43
Answer
45
Explanation
Solution
- Identify cathode (Fe3+/Fe2+) and anode (I2/I−) half-reactions.
- Determine the number of electrons transferred (n=2).
- Calculate the standard potential for Fe3+/Fe2+ using given potentials relative to Fe(s). EFe3+/Fe2+o=3−23×Eo(Fe3+/Fe)−2×Eo(Fe2+/Fe)=13×(−0.036 V)−2×(−0.440 V)=0.772 V
- Calculate the standard cell potential (Ecello=Ecathodeo−Eanodeo). Ecello=0.772 V−0.539 V=0.233 V
- Calculate the standard Gibbs free energy change (ΔrGmo=−nFEcello). ΔrGmo=−(2)×(96500 C)×(0.233 V)=−44989 J=−44.989 kJ
- Take the magnitude and round to the nearest integer. ∣−44.989 kJ∣≈45 kJ
