Question
Question: For the molecule \[{\text{Xe}}{{\text{F}}_{\text{2}}}\]: State the geometry of the molecule?...
For the molecule XeF2:
State the geometry of the molecule?
Solution
As we know that in chemistry, periodic tables play a vital role. In the periodic table there are totally 118 elements. In the periodic table there are totally 18 columns and 7 rows. The columns are called groups. Hence, 18 groups in the periodic table. The rows are called periods. Hence, totally 7 period in the table. Xenon and fluorine are one of the elements in the periodic table. The symbol of xenon is Xe. The symbol of fluorine is F. Xenon is one of the noble gases in the periodic table. Fluorine is one of the halogens in the periodic table.
Complete answer:
We have to know that the molecular formula of xenon difluoride is XeF2.
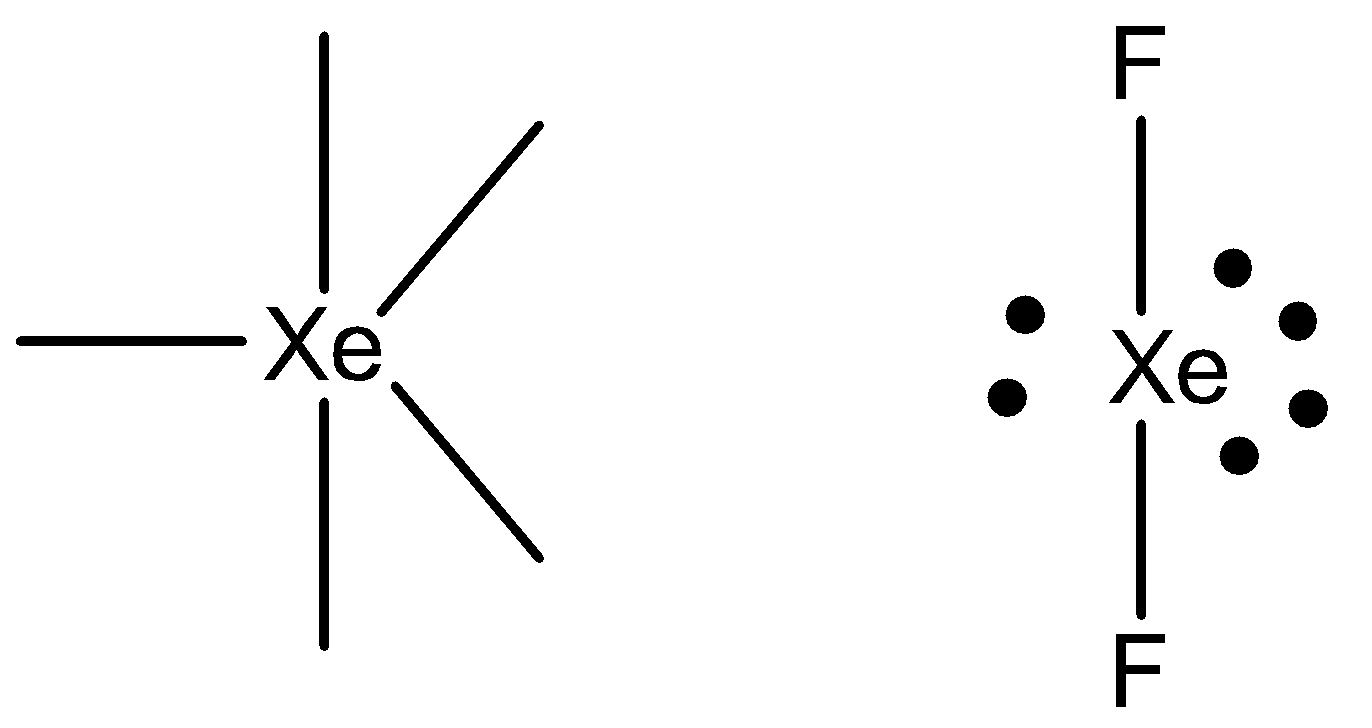
Xenon has an initial four lone pairs of electrons. Totally xenon having eight electrons in the valence shell. Out of these eight electrons, six electrons are in three lone pairs of electrons in such a way. But the remaining two electrons are making covalent bonds with two fluorine atoms. That is the reason for the formation of xenon difluoride.
The hybridisation of xenon difluoride is sp3d . The nature of geometry of sp3 d is bipyramidal structure.
Three lone pairs of electrons occupy an equatorial position in the trigonal bipyramidal structure.Two fluorine atoms occupy an axial position in the trigonal bipyramidal structure.
Hence, the geometry of the molecule xenon difluoride is linear.
The structure of the molecule xenon difluoride is given below,
Note:
We have to know that the atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons. The atomic number of xenon is 54. The atomic number of fluorine is 9. The mass number of xenon is 131.293. The mass number of fluorine is 18.99.
