Question
Question: For the given three step endothermic reaction, which of the following statement(s) is/are correct: ...
For the given three step endothermic reaction, which of the following statement(s) is/are correct:
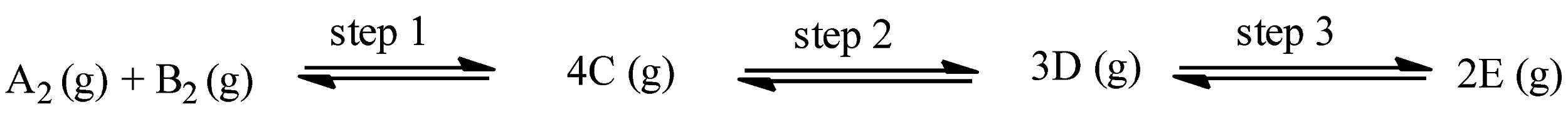
(a)- Step 1 is favored by high temperature and low pressure
(b)- Step 2 is favored by low temperature and high pressure
(c)- Step 2 is favored by high pressure and high temperature
(d)- Step 3 is favored by high pressure and high temperature
Solution
With the help of endothermic or exothermic property we can calculate the temperature required for the reaction, and for calculating the pressure of the reaction, a change in the number of moles can be seen. Both the factors are calculated according to Le Chatelier’s principle.
Complete answer:
We can solve this question according to Le Chatelier’s principle. So, we have to tell the conditions of temperature are the pressure that must be applied to the reaction, so that forward reaction is preferred.
The given reaction is:
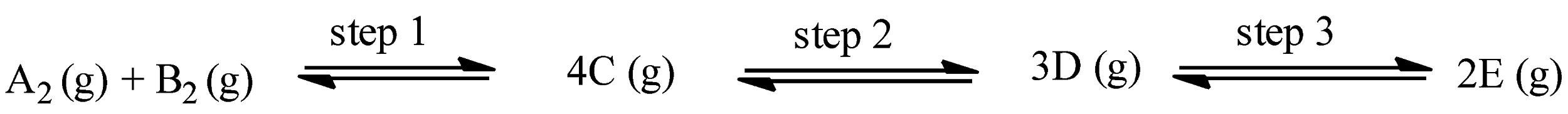
With the help of endothermic or exothermic property, we can calculate the temperature required for the reaction, and for calculating the pressure of the reaction, a change in the number of moles can be seen.
As it is stated that all the steps in the reaction are endothermic reactions, therefore, all the steps will move forward at high temperatures.
In step 1, the number of moles on the product side is 4 and the number of moles on the reactant side is 2, therefore,
Δn=4−2=2
So, Δn>1, this step will occur at low pressure.
In step 2, the number of moles on the product side is 3 and the number of moles on the reactant side is 4, therefore,
Δn=3−4=−1
So, Δn<1, this step will occur at high pressure.
In step 3, the number of moles on the product side is 2 and the number of moles on the reactant side is 3, therefore,
Δn=2−3=−1
So, Δn<1, this step will occur at high pressure.
So, corresponding to the above discussion, options (a), (c), and (d) are correct answers.
Note:
If the reaction given is exothermic then the low temperature is favored for the forward reaction, when the reaction given is endothermic the high temperature is favored for the forward reaction.
