Question
Question: For the given reaction \(N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O\overset{\Delta }{\mathop{\to }}\,...
For the given reaction Na2B4O7.10H2O→ΔX→ΔY+Z
X, Y and Z in the reaction are:
A. X=Na2B4O7, !! !! Y=NaBO2,Z=B2O3
B. X=Na2B4O7, !! !! Y=B2O3,Z=H3BO3
C. X=Na2B4O7 ,Y= !! !! NaBO2,Z=B(OH)3
D. X=Na2B4O7,Y= !! !! B2O3,Z= !! !! B(OH)3
Solution
Hint: When a compound possesses water of crystallisation on heating it first loses the water of crystallisation and on further heating it dissociates into its constituents. Same it with the given compound borax.
Complete answer:
When heated, borax undergoes various transitions which are mentioned as follows: It first loses 10 water molecules which is water of crystallisation and swells up. Then, it turns into a transparent liquid, solidifying to form a glass-like material which is called borax bead. If borax, Na2B4O7.10H2O is heated more on the platinum loop, then, a transparent colourless glass like bead of sodium metaborate (NaBO3) and boric anhydride (B2O3) is formed.
Na2B4O7.10H2O→ΔNa2B4O7→Δ2NaBO2+B2O3
So, answer is option A which is X=Na2B4O7, !! !! Y=NaBO2,Z=B2O3
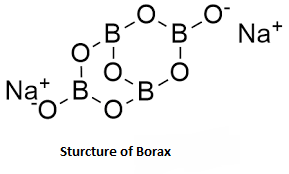
Note: Borax, also called sodium tetraborate, is a powdery white mineral that has been used as a cleaning product for several decades. It has many uses: It helps get rid of stains, mould, and mildew around the house. It can kill insects such as ants. It is an important boron compound, a mineral, and a salt of boric acid. Powdered borax is white, consisting of soft colourless crystals that dissolve easily in water. Borax has a wide variety of uses.
