Question
Question: For the given reaction 
Find the products A and B.
A. A:CH3CH2CH3,B:CH3CH2CH2Cl
B. A:CH3CH=CH2,B:CH2ClCH=CH2
C. A:CH2=CH2,B:CH3CH2Cl
D. A:CH3CH2CH3,B:CH3CH=CH2
Solution
We know that products can be deduced by using the formula or structure of reactant and the suitable reagent and/or conditions for every reaction would have a definite product. Alcohols show dehydration reaction with conc. H2SO4 at high temperatures.
Complete answer
We are given a reactant with formula, CH3CH2CH2OH. As we can see that it contains three carbons attached in a straight chain and there is one −OH group attached to the first carbon. We know that the name or category of a given organic compound can be deduced based on the functional group present in it by following the IUPAC guidelines for the same. According to the guidelines, −OH group is also a functional group and it gives rise to the “alcohols” category of organic compounds. So, here, the given reactant’s name can be deduced as propanol.
Now, we will have a look at the chemical reactions of alcohols for the presence of −OH group renders characteristic chemical properties to them. The first reagent that is given is conc. sulfuric acid and the reaction is being carried out at 170∘C. We know that alcohols undergo dehydration when treated with conc. H2SO4 at high temperatures as per the following chemical equation:
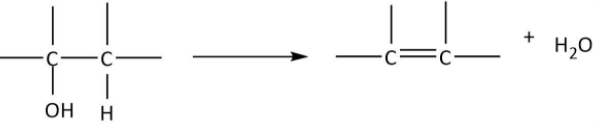
As it can be observed that hydroxyl groups along with one H from the adjacent carbon get removed as water and a double bond is formed between the two carbons. We can also write the chemical equation for propanol in the similar manner and deduce the formula of product A:
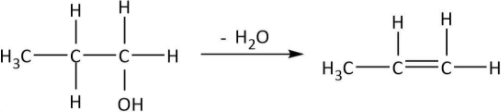
So, the product from dehydration (A:CH3CH=CH2) is an alkene. Now, it has been given that CH3CH=CH2 is treated with chlorine at 500∘C. The reaction can be shown by the following equation:
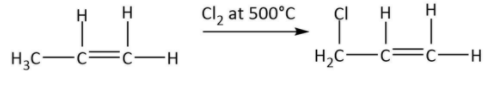
We know that reaction with chlorine at high temperature results in allylic chlorination and thus we get the product B:CH2ClCH=CH2.
**Hence, the correct option is B.
Note: **
We have to take into account the reaction conditions as well along with the reagents to deduce the product. The temperature and concentration of H2SO4 for obtaining is a necessary condition for the dehydration process and should not be altered.
