Question
Question: For the given orbital in column \[{\rm{I}}\], the only CORRECT combination for any hydrogen like spe...
For the given orbital in column I, the only CORRECT combination for any hydrogen like species is-
| Column 1 | Column 2 | Column 3 |
|---|---|---|
| (I) 1sorbital | {\rm{(i)}}$$$${{\rm{\varphi }}_{{\rm{n,l,}}{{\rm{m}}_{\rm{l}}}}}\propto {\left( {\dfrac{{\rm{Z}}}{{{{\rm{a}}_{\rm{0}}}}}} \right)^{\dfrac{{\rm{3}}}{{\rm{2}}}}}{{\rm{e}}^{\rm{ - }}}^{\left( {\dfrac{{{\rm{Zr}}}}{{{{\rm{a}}_{\rm{0}}}}}} \right)} | (P) 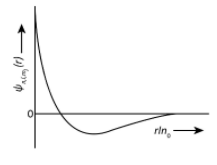 |
| (II) 2sorbital | (ii)one radial node | (Q) Probability density at nucleus |
| (III) 2pzorbital | {\rm{(iii)}}$$$${{\rm{\varphi }}_{{\rm{n,l,}}{{\rm{m}}_{\rm{l}}}}}\propto {\left( {\dfrac{{\rm{Z}}}{{{{\rm{a}}_{\rm{0}}}}}} \right)^{\dfrac{5}{{\rm{2}}}}}{\rm{r}}{{\rm{e}}^{\rm{ - }}}^{\left( {\dfrac{{{\rm{Zr}}}}{{{\rm{2}}{{\rm{a}}_{\rm{0}}}}}} \right)}{\mathop{\rm co}\nolimits} {\rm{s\theta }} | (R) Probability density is maximum at nucleus |
| {\rm{(IV)}}$$$${\rm{3d}}_z^2orbital | {\rm{(iv)}}$$$${\rm{xy}}plane is a nodal node | (S) Energy needed to excite electrons from n=2 state to n=4 state is 3227times energy needed to excite electrons from n=2 state to n=6 state |
(A) {\rm{(IV)}}$$$${\rm{(iv)}}(R)
(B) {\rm{(III)}}$$$${\rm{(iii)}} (P)
(C) {\rm{(II)}}$$$${\rm{(ii)}}(P)
(D) {\rm{(I)}}$$$${\rm{(ii)}}(S)
Solution
As we know that hydrogen is the simplest atom containing only one electron. n,l,ml resembles the principal quantum number, azimuthal quantum number and magnetic quantum number respectively. Radial node is measured from nl values. The probability density of finding the electron is never 100% because electrons are having Heisenberg uncertainty principle.
Complete answer
We will see the column one by one
In column 1, we will determine the radial nodes of the given orbitals by the formula
radialnode=n−l−1
For 1sorbital
radialnode=1−0−1=0
For 2sorbital
radialnode=2−0−1=1
For 3pzorbital
radialnode=3−1−1=1
For 3dz2orbital
radialnode=3−2−1=0
Now we could see from above explanation, the radial nodes is one for 2s and 3pz orbitals but the graph for 2sorbital is shown as
By the formula
Appling above formula we have values along xand y direction as-
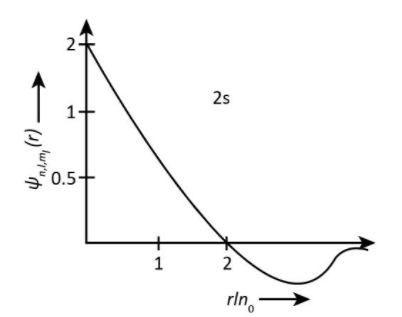
And the graph for 3pzorbital is shown as-
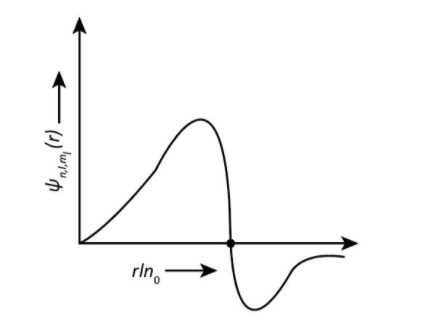
**Therefore, our correct option is option (C) -{\rm{(II)}}$$$${\rm{(ii)}}(P).
Note: **
φn,l,ml∝(a0Z)23e−(a0Zr) belongs to orbital 1s where principle quantum number can be calculated by e−(a0Zr) and azimuthal quantum number is calculated by minimum power of r or maximum power of sinθcosθ.
Energy of one- dimensional box is calculated by –
Ex=8ml2nx2h2
