Question
Question: For the given compounds: A) Tetracyanoethylene B) Carbon dioxide C) Benzene D) \[1,3 - \] Bu...
For the given compounds:
A) Tetracyanoethylene
B) Carbon dioxide
C) Benzene
D) 1,3− Butadiene
Ratio of σ and π -bonds is in order:
1.A = B > C < D
2.A = B > D < C
3.A = B = C D
4.D > C > B = A
Solution
We have to know that the single bonds means sigma bonds. Sigma (σ) bonds are not very strong bonds. Double bonds are strong bonds and they are the pi (π) bonds. It is difficult to break this bond. The ratio should always be written on reduced form. When we write ratios in reduced form then, we have more clarity. With the help of this method it is easy to find out the ratio of various compounds.
Complete step by step answer:
To count the bonds all one needs to know are the following rules of chemistry:
Single bond is 1σ bond.
Double bond is when there is one sigma (σ) and one pi (π) bond.
Triple bond is when there is one sigma (σ) and two pi (π) bonds.
A.We can draw the structure of Tetracyanoethylene as,
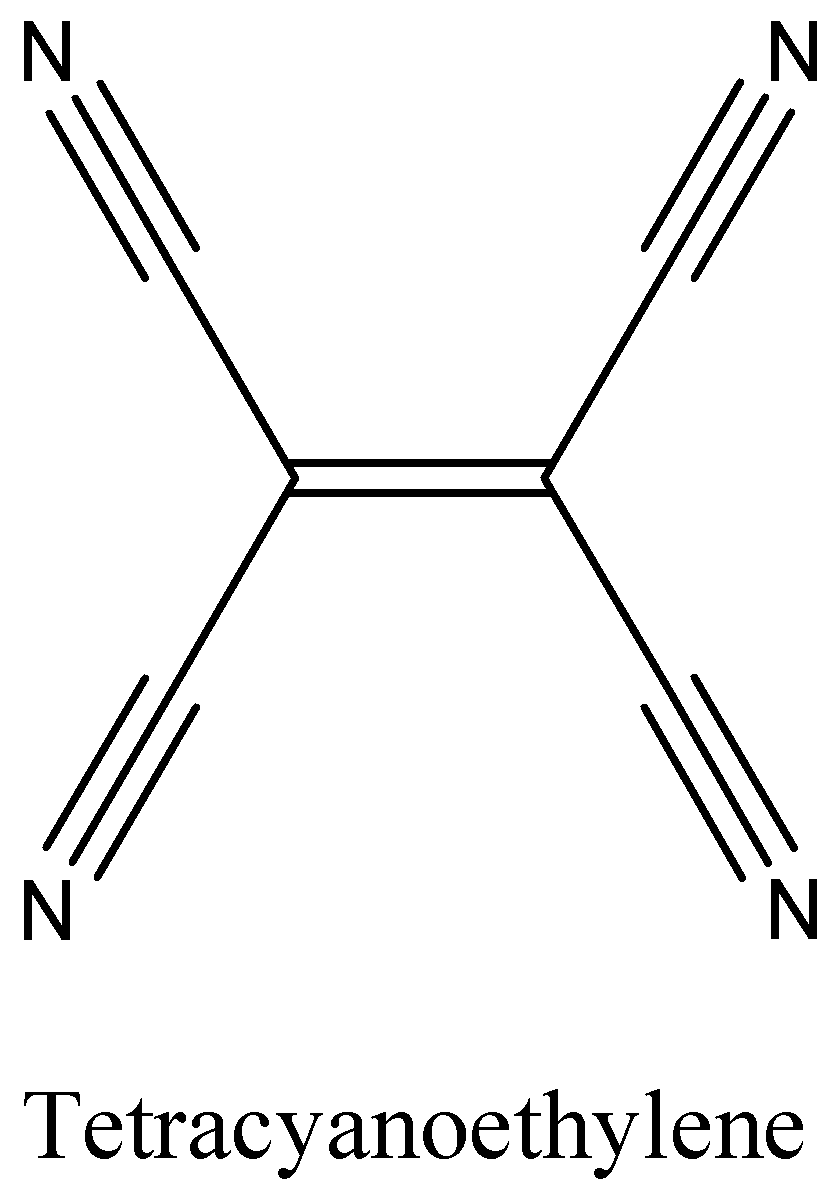
The above diagram clearly shows it has 9π bonds and 9σ bonds.
(B) We can draw the structure of Carbon dioxide as,
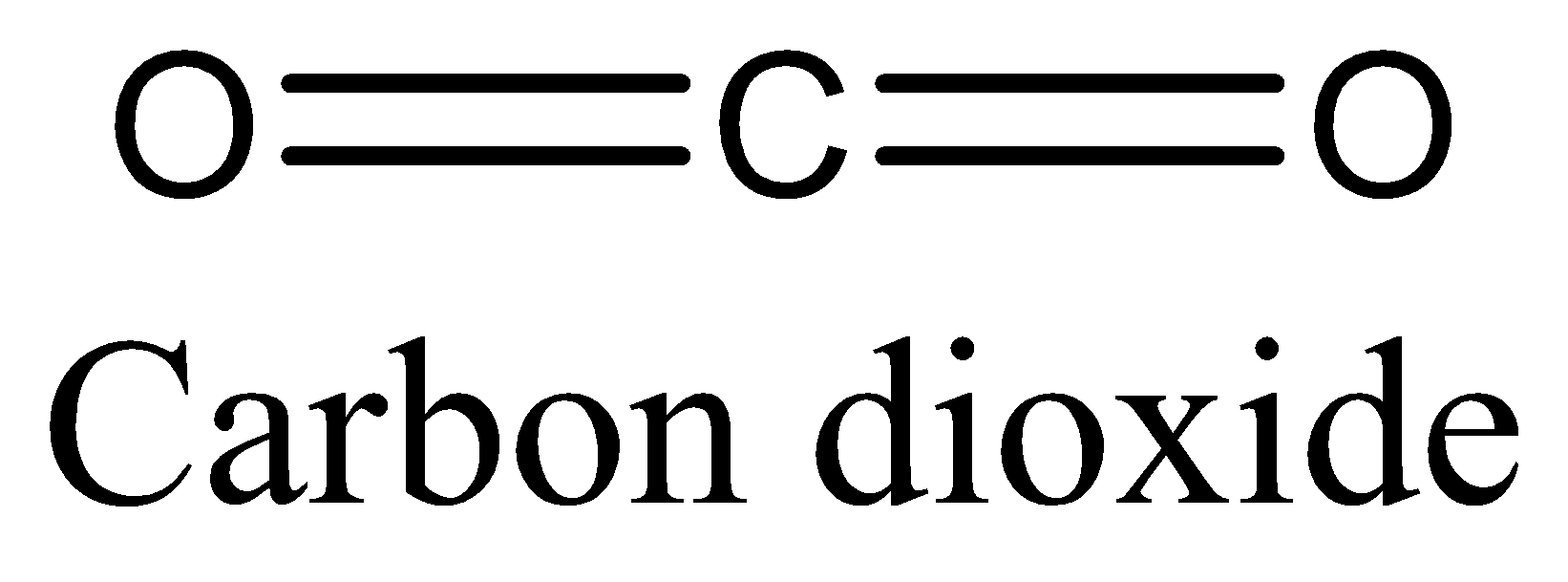
The above diagram clearly shows that carbon dioxide has 2π bonds and 2σ bonds.
(C) Now we can draw the structure of Benzene as,
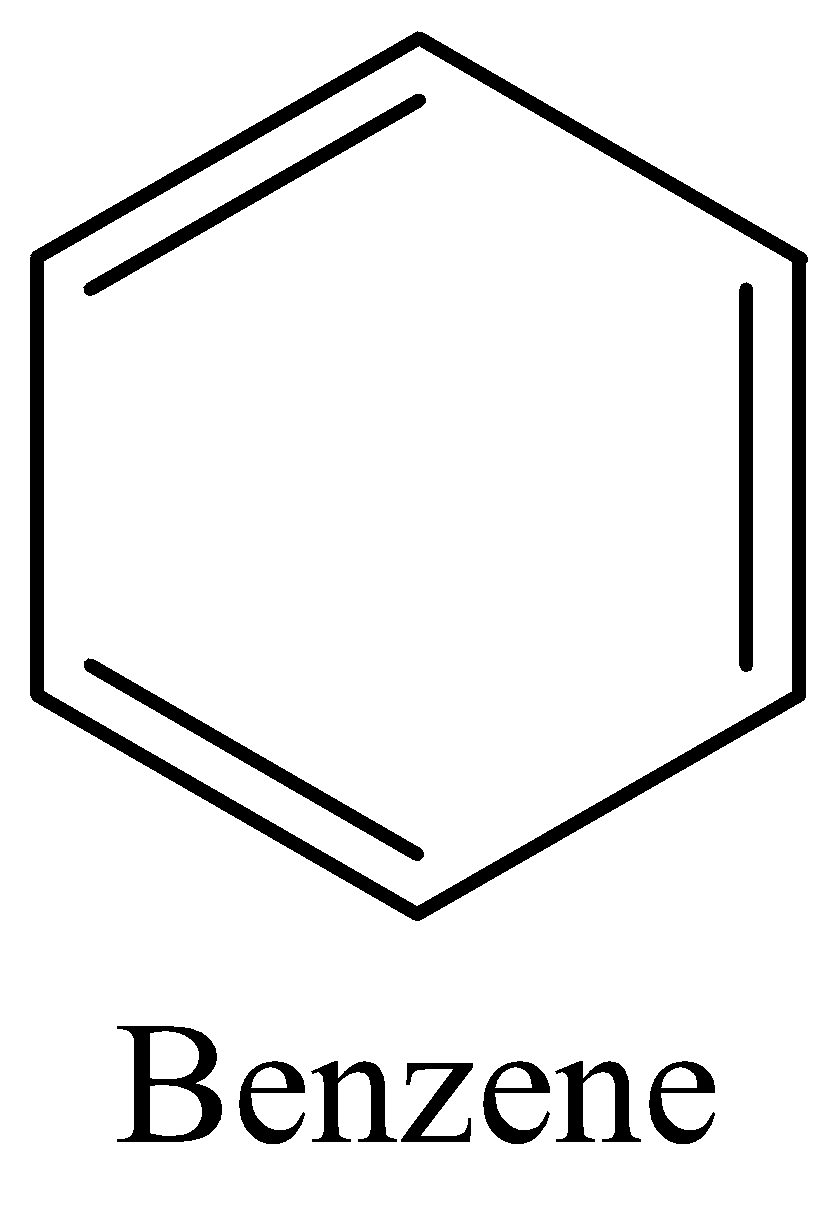
The above diagram clearly shows that the carbon dioxide has 3π bonds and 12σ bonds.
(D) Now we can draw the structure of 1,3-Butadiene as,
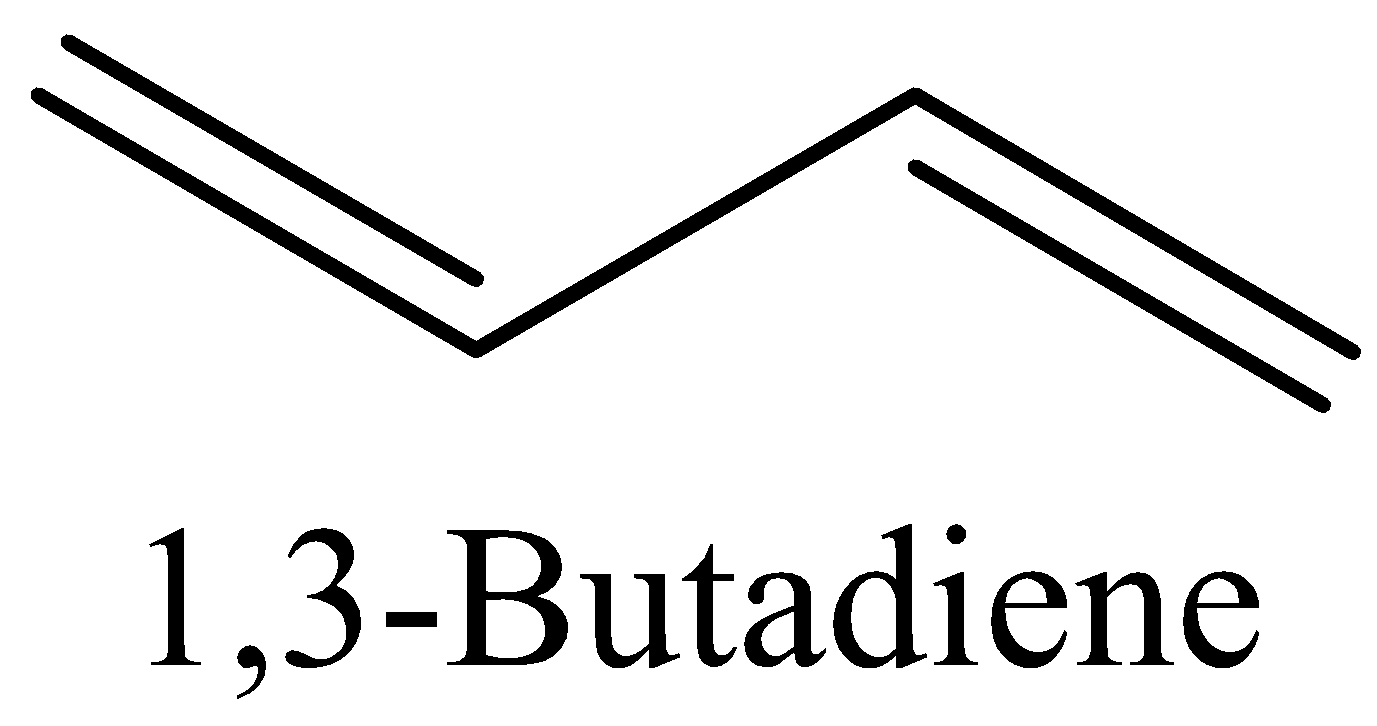
The above structure of 1,3-butadiene has 9π bonds and 2σ bonds.
From the above discussion we can conclude as,
In 1,3-Butadiene the ratio is 9:2
In benzene the ratio is 12:3 which is 4:1
In Carbon dioxide the ratio is 2:2 which is 1:1
In Tetracyanoethylene the ratio is 9:9 which is 1:1
Thus, D>C>B=A.
From this information we can conclude that Tetracyanoethylene and Carbon dioxide have the same ratio and benzene’s ratio is more than them. 1,3-Butadiene has the highest ratio.
So option 4 is correct for the given question.
Note:
We also remember that benzene is a compound which is a cyclic compound. It has alternate double and single bonds. Carbon dioxide has a carbon attached to two different oxygen. Tetracyanoethylene is colourless in nature and it is also important in organic chemistry. 1,3-Butadiene is a colourless gas which can be easily condensed into liquid state.
