Question
Question: For the formation of \[MgC{l_2}\] , which of the following are correct ? (A) \[Mg\] loses two elec...
For the formation of MgCl2 , which of the following are correct ?
(A) Mg loses two electrons
(B) Each Cl atom gains 2 electrons
(C) Each Cl atom gains one electron
(D) Mg and Cl shares electron to gain stable octet configuration
Solution
In the given question firstly we have to define the compound which is called Magnesium chloride. The chemical formula would be MgCl2 and the nature fo the bond formation would be ionic. Therefore we would be getting the bond formed as
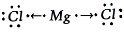
Complete step-by-step answer: The given question statement asks about the compound which is called Magnesium chloride. Apart from that we also have to know the nature of the bond formation and the elemental knowledge of the elements.
When we talk about the elements as an individual they show this kind of structure
For the case of chlorine it is :
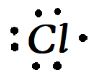
For the case of magnesium it is :
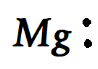
So when we talk about the bond formation we get that the ionic bond that is formed between the magnesium and the chlorine would be ionic were the one will lose the electrons in order to et the octet to be filled whereas the chlorine would be there to gain the solo electron and get the octet filled.
Therefore the transfer of the electron would be completely depicted in the given image of the bond formation.
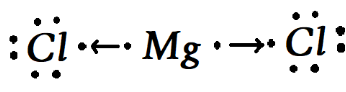
So therefore we get two conclusions that the
1.The Magnesium will loose couple of electrons
2.The Chlorine would going to receive an electron
Therefore the right options among the following would be :Option A, Mg loses two electrons.
Option C, Each Cl atom gains one electron.
Note: Some magnesium chloride is made from solar evaporation of seawater. Anhydrous magnesium chloride is the principal precursor to magnesium metal, which is produced on a large scale. Hydrated magnesium chloride is the form most readily available.
