Question
Question: For the formation of ammonia from N₂ and H₂, if rate of disappearance of N₂ is 2 M/s, then the rate ...
For the formation of ammonia from N₂ and H₂, if rate of disappearance of N₂ is 2 M/s, then the rate of appearance of NH₃ and the rate of reaction respectively would be:
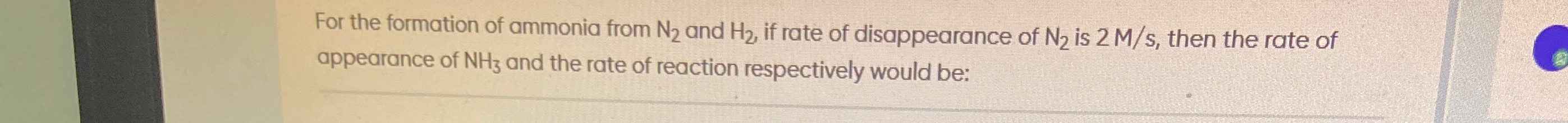
4 M/s and 2 M/s
2 M/s and 4 M/s
1 M/s and 2 M/s
4 M/s and 4 M/s
4 M/s and 2 M/s
Solution
The balanced chemical equation for the formation of ammonia from N₂ and H₂ is:
N₂(g) + 3H₂(g) → 2NH₃(g)
The rate of reaction in terms of the disappearance of reactants and appearance of products is given by:
Rate of reaction = −11dtd[N2]=−31dtd[H2]=+21dtd[NH3]
Given: Rate of disappearance of N₂ = −dtd[N2] = 2 M/s
1. Calculate the Rate of Reaction:
From the definition, the rate of reaction is equal to the rate of disappearance of N₂ (since its stoichiometric coefficient is 1).
Rate of reaction = −dtd[N2]
Rate of reaction = 2 M/s
2. Calculate the Rate of Appearance of NH₃:
We relate the rate of reaction to the rate of appearance of NH₃:
Rate of reaction = +21dtd[NH3]
Substitute the calculated rate of reaction:
2 M/s = +21dtd[NH3]
Multiply both sides by 2 to find the rate of appearance of NH₃:
dtd[NH3] = 2 × 2 M/s
dtd[NH3] = 4 M/s
Therefore, the rate of appearance of NH₃ is 4 M/s, and the rate of reaction is 2 M/s.
