Question
Question: For the following two statements, choose the correct option: Assertion: Ethyl chloride with ethano...
For the following two statements, choose the correct option:
Assertion: Ethyl chloride with ethanolic AgCN gives ethyl isocyanide as a major product.
Reason: In ethyl cyanide, carbon of CN group is sp hybridized.
A. Both assertion and Reason are true and Reason is the correct explanation for Assertion
B. Both assertion and Reason are true and Reason is not the correct explanation for Assertion
C. Assertion is true and Reason is false
D. Assertion is false but Reason is true
Solution
We know that isocyanide is an organic compound which possesses the functional group −N≡C. This functional group is isomer to the nitrile −C≡N group. In nitrile, the alkyl group is bonded to C atom whereas in isocyanide, the alkyl group is bonded to N atom.
Complete step by step answer:
Now, we discuss the reaction of ethyl chloride with ethanolic AgCN. In this reaction, a carbocation forms first. The formation of carbocation can be shown as below.
CH3CH2Cl+AgCN→CH3CH2++AgCl
In the next step, electrophilic attack of CN− occurs to form ethyl isocyanide as major product.The reaction is,
CH3CH2++NC−→CH3CH2NC
As we see the ethyl group is bonded to the N atom of the CN group. So, it is an isocyanide. Therefore, we can say that assertion is correct.
Let’s check whether the second statement is correct or not. It says, hybridization of C of CN group is sp.
We know that hybridization is the phenomenon of mixing of orbitals of same atom with slight difference in energies so as to redistribute their energies and give new orbitals of equivalent energy and shape.
There is a method to check the hybridization of atoms.
- If four electron groups surrounded the atom, then hybridization is sp3
. - If three electron groups surrounded the atom, then hybridization is sp2
. - If two electron groups surrounded the atom, then hybridization is sp
Now, we have to check the hybridization of C of CN in ethyl cyanide. The structure of ethyl cyanide is,
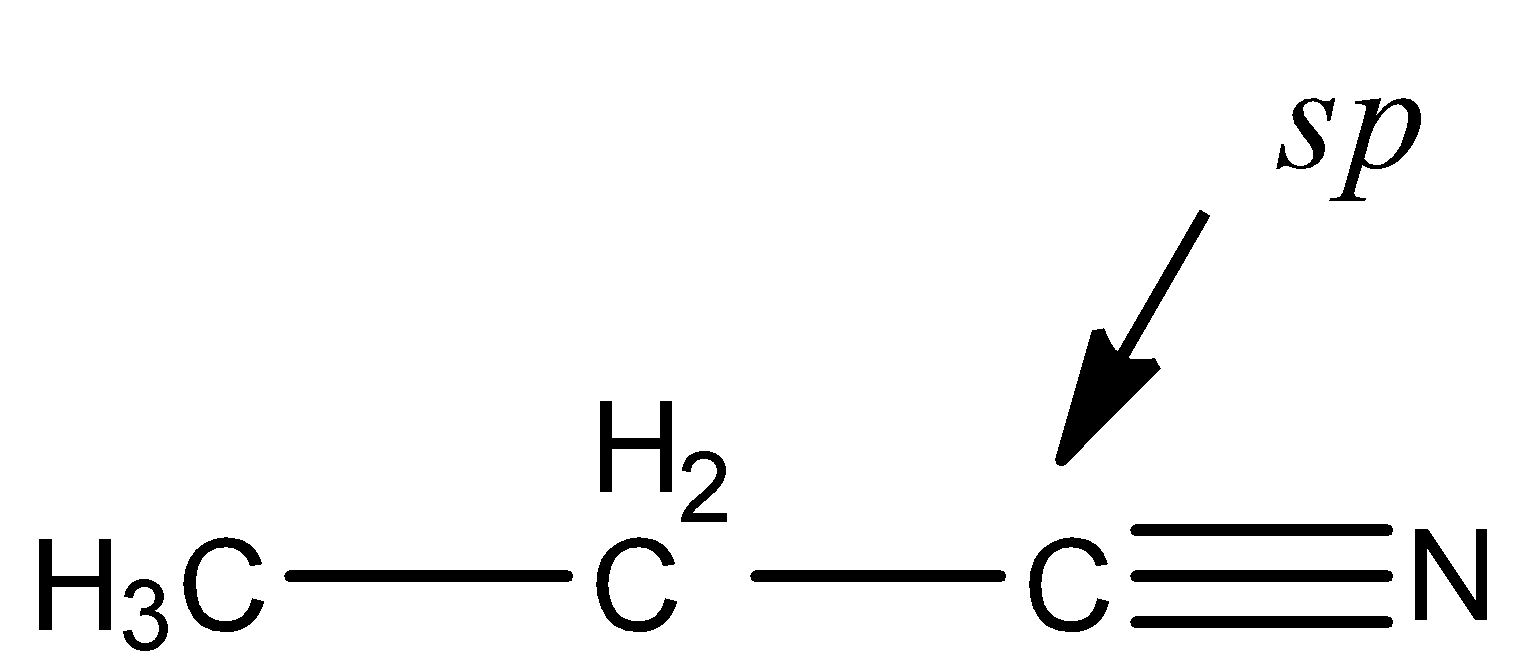
The C of CN group is sp hybridized as carbon is surrounded by two electron groups. So, we can say that, second statement is also correct. But there is no relation of the second statement (Reason) to the first statement.
Hence, the correct answer is option B.
Note:
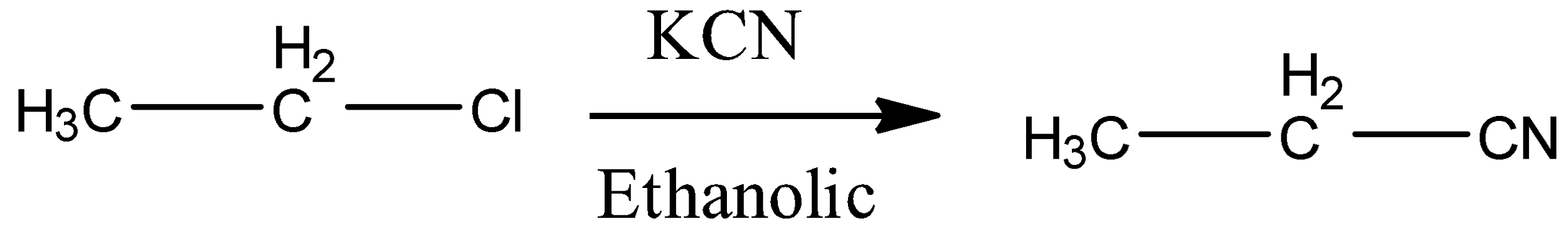
Always remember that reaction of ethyl chloride with KCN or NaCN results in ethane nitrile because the reaction is nucleophilic substitution reaction. The reaction can be shown as follows: