Question
Question: For the following reactions: Which of the following statements is correct? \( C{{H}_{3}}C{{H}_{2}...
For the following reactions: Which of the following statements is correct?
CH3CH2CH2Br+KOH→CH3CH=CH2+KBr+H2O
(a) CH3CH2CH2Br+KOH→CH3CH=CH2+KBr+H2O
(b) 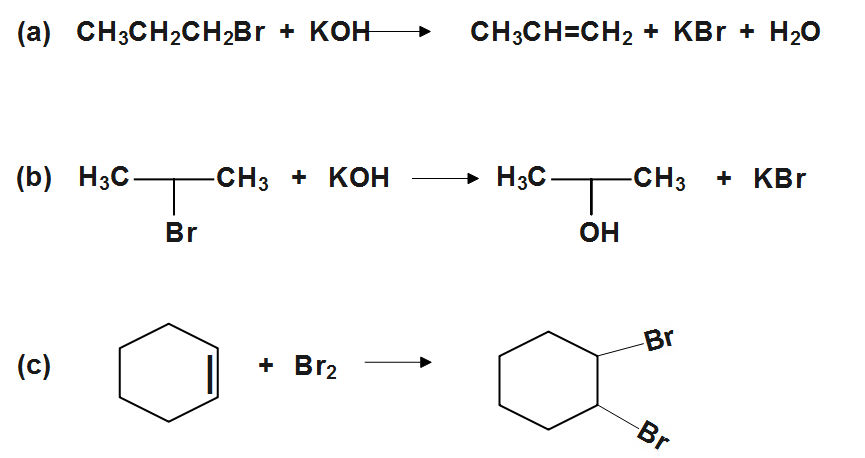
(c) 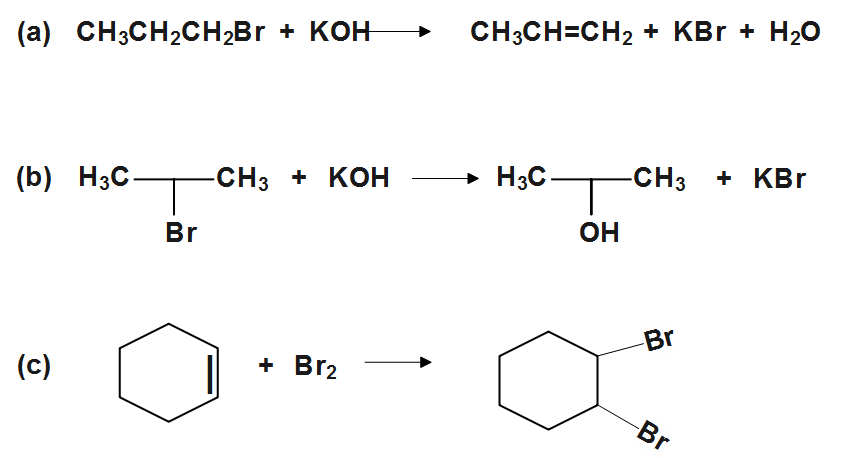
(A) (a) and (b) are elimination reactions and (c) is an additional reaction.
(B) (a) is elimination, (b) is substitution and (c) is an additional reaction.
(C) (a) is elimination, (b) and (c) are substitution reactions.
(D) (a) is substitution, (b) and (c) are additional reactions.
Solution
Hint : We know that the addition reaction occurs when two or more reactants combine to form a single product. This item will contain all the molecules that were available in the reactants. Additional responses happen with unsaturated compounds. A disposal response happens when a reactant is separated into two products.
Complete Step By Step Answer:
Here (A) is eliminated, When an alkyl bromide is heated with alcoholic KOH solution, a molecule of HBr is eliminated to form C=C double bond. Since elimination reaction is something that comes off of a molecule, resulting in two products. A→B+C
Similarly (B) is a substitution reaction which when secondary alkyl bromide is heated with aqueous KOH , bromine atom is substituted with −OH group. Since substitution reaction involves the exchange of one group for another. AB+CD→AC+BD
Similarly (C) is an additional reaction, a molecule of bromine is added across C=C double bond. Since the additional reaction is something is added to something else to produce a third thing. A+B→C . The letters A, B and C here speak to any nuclear, ionic or sub-atomic species which can go through this kind of response.
Therefore, correct answer is option B i.e. (a) is elimination, (b) is substitution and (c) is addition reaction.
Note :
Note that the substitution reaction is when you replace a single functional group with another. An option response is the point at which you add a practical gathering to a compound. A replacement response happens when a trade of components in the reactants takes place. The starting reactants are changed or swopped around to give an eventual outcome.
