Question
Question: For the following reaction which of the following is/are correct? 
(a)- Both X and Y are five membered ring
(b)- Reaction (i) follows E2 path whereas reaction (ii) follows E1 path
(c)- Reaction (ii) has carbocation as an intermediate
(d)- Carbocation rearrangement takes place in reaction (i)
Solution
In both reactions, there will be a formation of alkene because there is an elimination reaction and there will be the removal of water molecules. The reaction in which carbocation is formed will be the E1 path and the other will follow the E2 path.
Complete answer:
The given reactant in the reactions is 1- cyclobutylethanol.
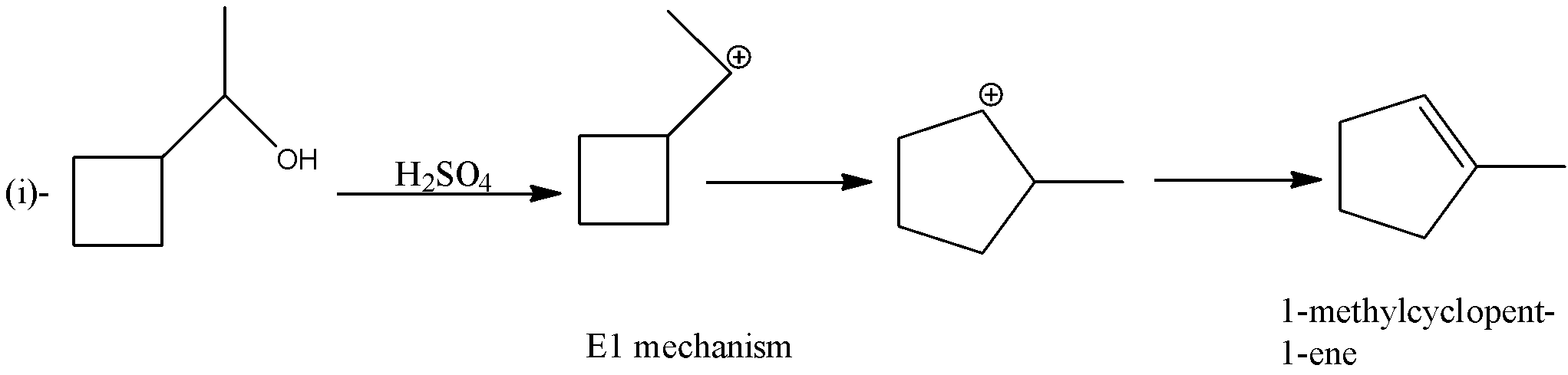
In the first reaction, 1- cyclobutylethanol is treated with sulfuric acid, so the hydroxyl ion will be removed which will form a carbocation. There will be rearrangement of carbocation and there will be the formation of alkene. The product formed will be 1-methylcyclopent-1-ene. The reaction is given below:
In the second reaction, there will be the formation of ethylene cyclobutane and there will be no formation of a carbocation. The reaction is given below:
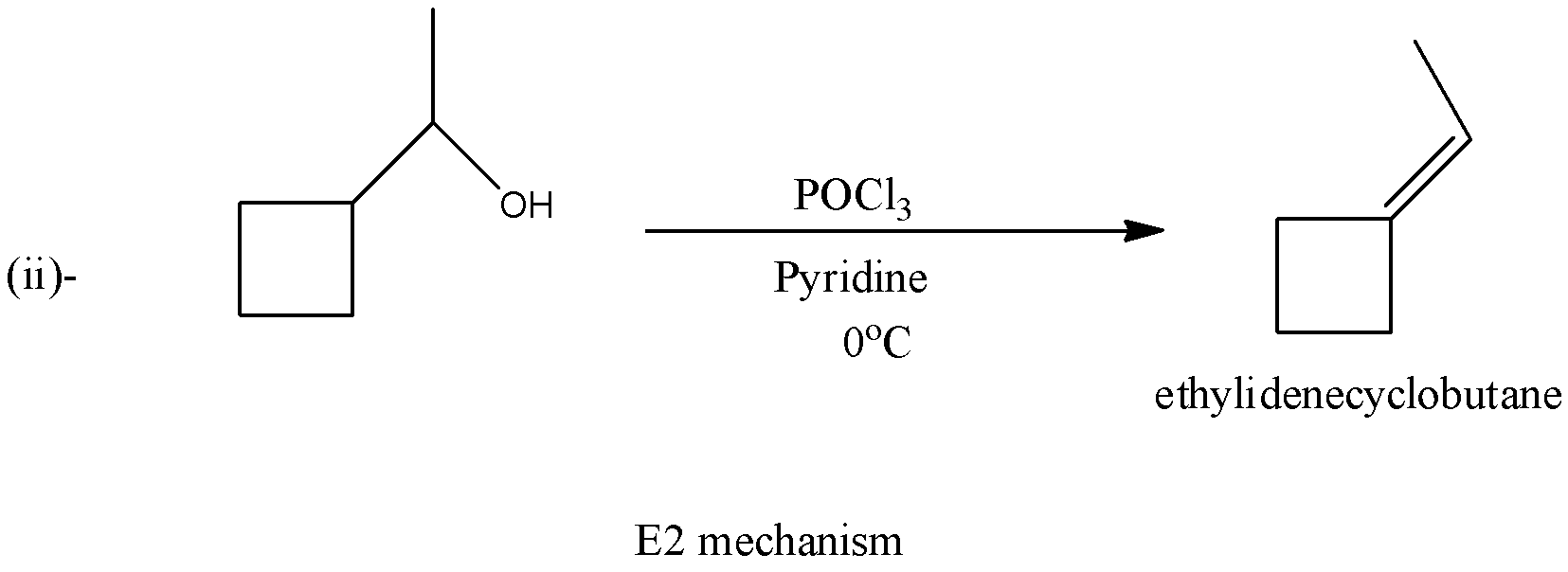
So, as we can see, in the first reaction there are five membered rings and in the second reaction, there is a four-membered ring. The first reaction follows the E1 mechanism and the second reaction follows the E2 mechanism.
Therefore, from the given options only option (d)- Carbocation rearrangement takes place in reaction (i) is correct in the given reactions.
Hence, the correct answer is an option (d).
Note:
The rearrangement of carbocation will take place only when there is a possibility of a more stable carbocation. A tertiary carbocation is most stable while primary carbocation is the least stable.
