Question
Question: For the following reaction product is: \({{\text{H}}_{\text{3}}}{\text{C}} - {\text{CH}}\,{\text{ ...
For the following reaction product is:
H3C−CH = CH2+HCl→
Solution
Hydrogen halide gives an addition reaction to alkene. Alkene behaves as nucleophilic so, gets protonated by hydrogen halide. Then at carbocation halide attacks. So, alkyl halide forms as a product. The formation of the product is decided based on the Markovnikov rule.
Complete step by step solution:
The reaction of hydrogen chloride with alkene is an example of an electrophilic addition reaction. In the first step, the electrophilic addition takes place. In the second step, the nucleophilic addition takes place.
Hydrogen chloride gives an addition reaction on propene. During the reaction, first, alkene attacks on the hydrogen of hydrogen chloride, so a carbocation forms. On this carbocation, chloride gets attached.
According to the Markovnikov rule, the negative part of the attacking group gets attached to the position where less number of hydrogens are present.
The product formation is shown as follows:
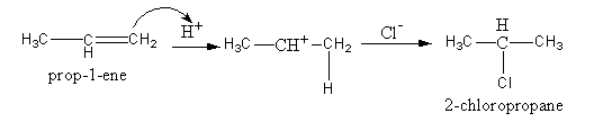
So, by the reaction of propene with hydrogen chloride, the product formed is 2− chloropropane. In product, the negative chloride ion is attached to carbon that has only one hydrogen atom.
Note: Markovnikov rule is based upon the observation of stability of the carbocation which formed during the reaction. In the case of unsymmetrical alkene two possibilities are there for the formation of a carbocation.
Which are shown as follows:
In secondary carbocation, the more electron-donating groups are present which stabilized the positive charge of the carbocation. So secondary carbocation is more stable than primary, so the reaction goes through the formation of a secondary carbocation.
The stability order of carbocation is as follows:
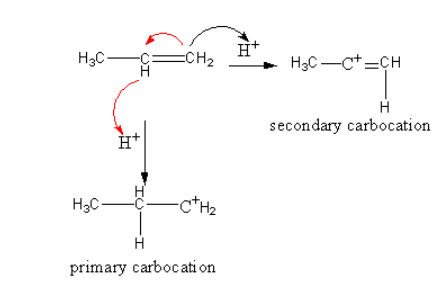
Tertiary carbocation > secondary carbocation > primary carbocation.
Marcovnikov's rule can also be stated as that hydrogen of the attacking group gets attached where hydrogens are present in more numbers.
