Question
Question: For the following molecules, the correct order of stability of organic intermediate formed by hetero...
For the following molecules, the correct order of stability of organic intermediate formed by heterolytic bond cleavage of carbon bromine bond is:
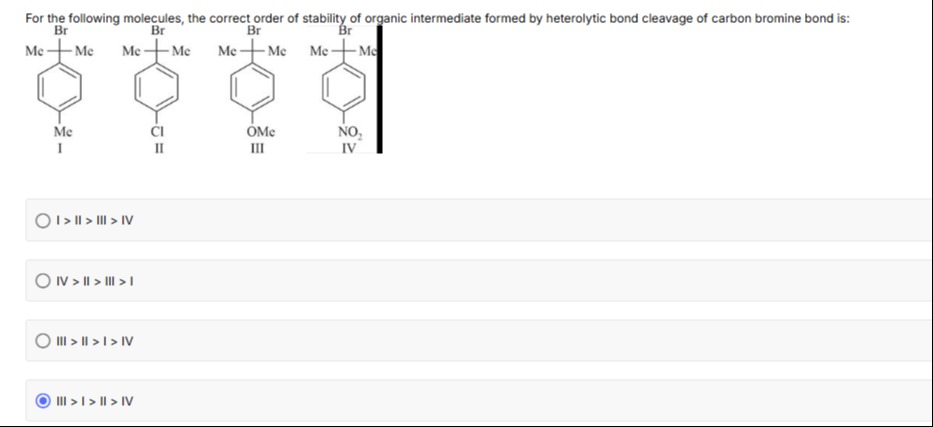
A
I > II > III > IV
B
IV > II > III > I
C
III > II > I > IV
D
III > I > II > IV
Answer
III > I > II > IV
Explanation
Solution
For a benzylic carbocation, stability is enhanced by groups that donate electron density via resonance. The methoxy group (OMe) in structure III strongly donates electrons (+M effect), stabilizing the adjacent carbocation more than the methyl (Me) group in I, which in turn is better than chlorine in II. The nitro group (NO₂) in IV is strongly electron withdrawing (–M effect) and destabilizes the carbocation.
Thus, the correct order is:
III>I>II>IVExplanation (minimal):
- III (OMe): strong +M donation stabilizes the cation.
- I (Me): weak +I electron donation.
- II (Cl): moderate -I (with some +M) less effective than Me.
- IV (NO₂): strong –M withdraws electron density, destabilizing the cation.
