Question
Question: For the following compounds, the correct statement (s) with respect to nucleophilic substitution rea...
For the following compounds, the correct statement (s) with respect to nucleophilic substitution reaction is are:
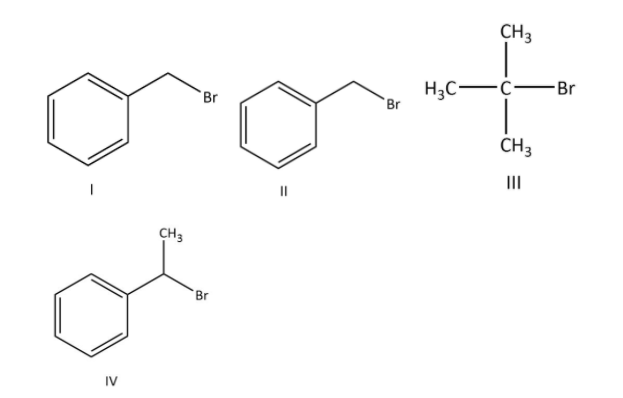
(A) I and III follow SN1 mechanism
(B) Compound IV undergoes inversion of configuration
(C) I and II follow SN2 mechanism
(D) The order of reactivity for I, III and IV is: I, III and IV>I>III.
Solution
As we know that, the nucleophilic substitution reaction is of two types, one is unimolecular nucleophilic substitution reaction and bimolecular nucleophilic substitution reaction. Unimolecular nucleophilic substitution reaction depends upon only the reaction intermediate and bimolecular nucleophilic substitution reaction depends upon nucleophile as well as substrate.
Complete step by step answer
Unimolecular nucleophilic substitution reaction is represented as SN1 and bimolecular nucleophilic substitution reaction represented as SN2.
SN1 reaction proceeds only when the carbocation intermediate is very stable. That’s why it is represented by one as it depends only on the carbocation intermediate. SN1 reaction mechanism is completed by two steps, in which first step is to generate carbocation intermediate and then attack of nucleophile. Even weak nucleophiles can approach the carbocation intermediate. The stability of carbocation in groups follows the order is benzylcarbocation>30>20>10.
SN2 reaction depends upon the concentration of the nucleophile and order of substrate. The kinetic order of the reaction is two and the mechanism is completed in only one step.
The order of the substrate in the alkyl group to attack the nucleophile is 10>20>30.
Now in the given options,
I and III follow SN1 mechanism because I and III will form most stable carbocation as we have seen in our explanation.
In option (B), the IV compound will undergo inversion in configuration by SN2 mechanism when the nucleophile approach to it.
In option (C), I and II will follow SN2 mechanism because both of the compounds contain primary carbon.
In option (D), the order of reactivity by SN1 mechanism for I, III and IV is IV>I>III as we have seen our explanation.
**Therefore, the correct options are (A), (B), (C) and (D).
Note: **
SN2 mechanism form products with inversion in configuration and SN1 mechanism form products with retention in configuration.
