Question
Question: For the complex \( \left[ {Fe{{\left( {CO} \right)}_x}} \right] \) , which of the following statemen...
For the complex [Fe(CO)x] , which of the following statements is wrong?
A. It is a σ−π bonded organometallic compound.
B. In the complex, the value of x=6 .
C. In the complex, CO is a π acid ligand.
D. It is trigonal pyramidal in shape.
Solution
Hint : We know that a coordination complex consists of transition metals which act as Lewis acid and accept electrons from ligands by coordinate bonds. An organometallic compound is defined as the compound in which the central metal atoms are directly bonded with the carbon atom of a molecule.
Complete Step By Step Answer:
To identify the wrong statement among given options, we need to understand the concept of each statement in brief as follows:
Statement-1: It is a σ−π bonded organometallic compound.
In metal carbonyls, i.e., the compound in which the metal atom is bonded to carbon monoxide, the bond possesses both the σ and π character. Due to donation of electrons of carbon monoxide to the vacant orbitals of metal, a metal-carbon σ bond is formed and due to the donation of a pair of electrons from a filled d-orbital of metal into the vacant anti bonding i.e., π∗ orbital of carbonyl ligands, a metal-carbon π bond is formed which is also referred to as back bonding.
Thus, [Fe(CO)x] is a σ−π bonded organometallic compound. Hence, it is a correct statement.
Statement-2: In the complex, the value of x=6 .
Effective atomic number (EAN) represents the total number of electrons present in a metal complex surrounding the nucleus of a metal atom. It is often referred to as the 18 electron rule and its value is equal to the atomic number of the nearest inert gas (36 for 3d transition metals). Thus, with the help of this rule, the number of ligands and products of the reaction can easily be determined. It is given by the formula:
EAN=Z+(l×n)−x
Where, Z is the atomic number of the metal, l is the number of ligands present, n is the number of electrons donated by one ligand and x is the oxidation state of the metal.
For the given metal complex [Fe(CO)x] , the required values are as follows:
| Variable | Value |
|---|---|
| Z | 26 |
| l | x |
| n | 2 |
| x | 0 |
So, according to EAN rule the value of x will be as follows:
26+2x−0=36
⇒x=5
Hence, the given statement is incorrect.
Statement-3: In the complex, CO is a π acid ligand.
Ligands which are capable of donating electrons to metal and form sigma bonds and also have a tendency to accept electrons from metals to their vacant anti-bonding orbitals are known as π acid ligands. Carbon monoxide i.e., CO is considered a π acid ligand because the filled sigma orbitals of CO donate electrons to the metal whereas the empty pi orbitals consist of a perfect symmetry to accept electrons via back bonding. Hence, the given statement is correct.
Statement-4: It is trigonal pyramidal in shape.
In [Fe(CO)5] , the Fe atom is dsp3 hybridized and the corresponding shape of the compound is trigonal bipyramidal which is represented as follows:
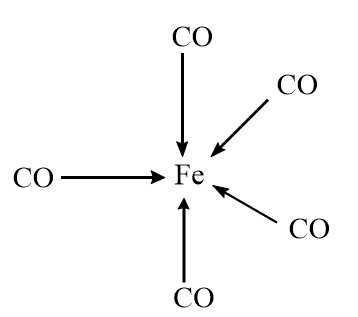
Hence, it is the correct statement.
Therefore, the correct answer for the given question is option (B).
Note :
It is important to note that the EAN rule can explain the stability of the metal carbonyls but it cannot be the only criteria to form stable carbonyls. The metals with an odd number of electrons will not obey the EAN rule because the total number of electrons will be odd no matter how many carbonyls are bonded to it.
