Question
Question: For the complex ion \[{[Fe{(CN)_6}]^{ - 3}}\], state: (A) The type of hybridisation (B) The magn...
For the complex ion [Fe(CN)6]−3, state:
(A) The type of hybridisation
(B) The magnetic behaviour
(C) The oxidation number of the central metal atom.
Solution
The strong field ligands in a compound cause large splitting while the weak field ligands in a compound cause small splitting. The large splitted compounds are low spin complexes while the small splitted compounds are high spin complexes.
Complete answer:
[Fe(CN)6]−3anion is known as ferricyanide. It is also known as hexacyanoferrate (III). The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant.
[Fe(CN)6]−3 consists of Fe3+ as the central atom bound in octahedral geometry to six cyanide ligands. The iron is low spin due to the presence of six strong field ligands. Compared to normal cyanides like potassium cyanide, ferrocyanides are much less toxic because of the tight hold of the cyanide ions to the iron centre. The structure of [Fe(CN)6]−3 is:
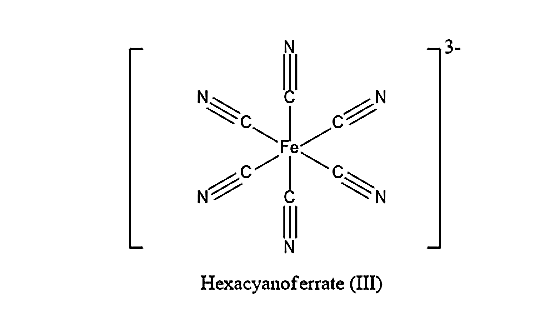
(A) In [Fe(CN)6]−3complex, the cyano group i.e., CN is a strong field ligand. Therefore, Cyano group being a strong field ligand will form a low spin complex using inner 3d orbitals. As there are six ligands present, it will form octahedral geometry. Therefore, the hybridisation present in [Fe(CN)6]−3is d2sp3.
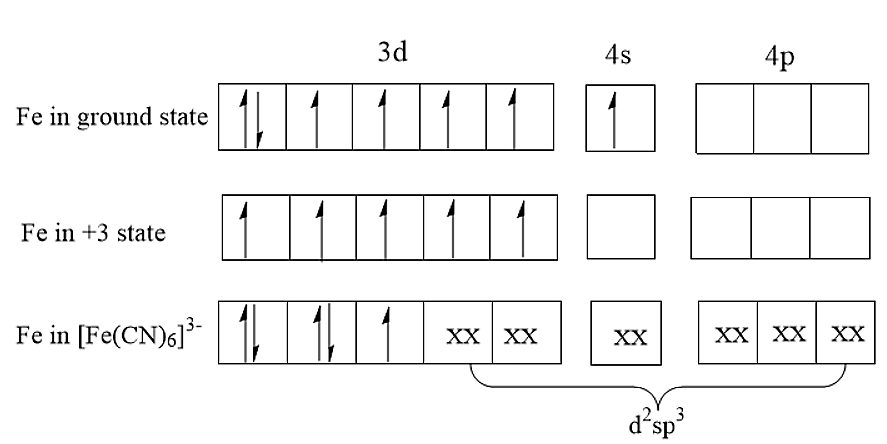
(B) The central atom has an oxidation number of +3. Thus, Fe3+has an unpaired electron. Thus, the complex will be paramagnetic.
(C) The oxidation number of the central metal atom is +3, which can be calculated by the equation
x + 6(-1) = -3
x = +3
Note:
Remember that the presence of one unpaired electron is sufficient for paramagnetism. Make sure to check the configuration of each atom for paramagnetism also, with the molecule as a whole.
