Question
Question: For the cell Zn(s) | Zn^{2+}(aq) || M^{x+}(aq) | M(s), different half cells and their SRP are given ...
For the cell Zn(s) | Zn^{2+}(aq) || M^{x+}(aq) | M(s), different half cells and their SRP are given below:
Couple: Au^{3+}/Au, Ag^{+}/Ag, Fe^{3+}/Fe^{2+}, Fe^{2+}/Fe
SRP: 1.40 V, 0.80 V, 0.77 V, –0.44 V
If E^{\circ}{Zn^{2+}|Zn} = –0.76 V, which cathode will give a maximum value of E^{\circ}{cell} per electron transferred?
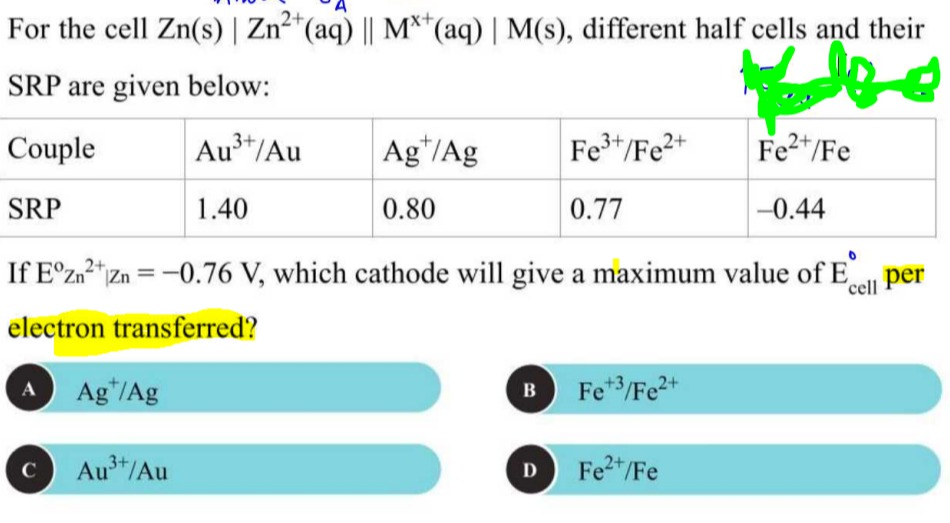
Ag^{+}/Ag
Fe^{+3}/Fe^{2+}
Au^{3+}/Au
Fe^{2+}/Fe
Ag^{+}/Ag
Solution
Cell potential is E^{\circ}{cell}=E^{\circ}{cathode}–E^{\circ}{anode}=E^{\circ}{cat}+0.76 V.
Compute per-electron value:
• Ag^{+}/Ag: E_{cell}=0.80+0.76=1.56 V, uses 2 e^– ⇒ 1.56/2=0.78 V per e^–
• Fe^{3+}/Fe^{2+}: 0.77+0.76=1.53 V ⇒ 1.53/2=0.765 V per e^–
• Au^{3+}/Au: 1.40+0.76=2.16 V, uses 6 e^– ⇒ 2.16/6=0.36 V per e^–
• Fe^{2+}/Fe: –0.44+0.76=0.32 V ⇒ 0.32/2=0.16 V per e^–
Ag^{+}/Ag gives the highest E^{\circ}_{cell} per electron transferred.
