Question
Question: For the calculation of molecular weight of dibasic acid using silver salt method, a straight line as...
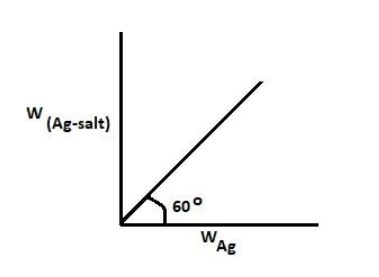
For the calculation of molecular weight of dibasic acid using silver salt method, a straight line as shown is obtained when a graph is plotted between weight of silver salt and weight of silver. Find the molecular weight of acid (Take 3 = 1.7)
A.76.6
B.8
C.153.2
D.0
Solution
Silver salt method is used for determining the molecular masses of carboxylic acids. This method is based on the fact that they form insoluble silver salts, which upon heating decompose to leave a residue of metallic silver.
Complete Step by step answer: Silver salt method for acids: It is based on the fact that silver salt of an organic acid on heating gives residue of metallic silver.
Knowing the equivalent mass of silver salt, the equivalent mass of the acid can be obtained. The molecular mass of an acid can be determined.
Molecular mass of acid in the silver method is given by the formula: MolecularMassofacid=[Wt.ofsilverWt.ofsilversalt×(108−107)]×BasicityOfAcid
So from the graph shown above we know that Wt.ofsilverWt.ofsilversalt=tan600
And we are also given that: tan600=3=1.7
As the question is for dibasic acid hence the basicity of acid = 2 (di means 2)
So, putting all these values in the formula for the molar mass of acid and solving MolarMassOfAcid=[1.7×(108−107)]×2 molarmassofacid=(183.6−107)×2=76.6×2=153.2
molarmassofacid=153.2gm.
Hence the correct option is (c).
Note: From the volume of the silver salt given and the mass of the silver residue obtained, the equivalent mass of the silver salt can be calculated. Any method of quantitative analysis in which the amount of a substance is determined by measuring the volume that it occupies the volume of a second substance that combines with the first in known proportions, more correctly called titrimetric analysis is called volumetric analysis.
