Question
Question: For reaction $CrO_2Cl_2 + NaOH \longrightarrow B + NaCl + H_2O$ $B + H^+ \longrightarrow C + H_2O$...
For reaction
CrO2Cl2+NaOH⟶B+NaCl+H2O
B+H+⟶C+H2O
Then number of terminal oxygen in C is ______
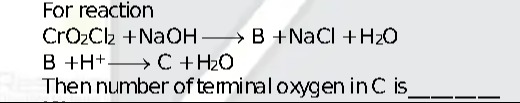
Answer
4
Explanation
Solution
The compound CrO2Cl2 (chromyl chloride) reacts with NaOH to form a chromate salt (B), which upon acidification gives chromic acid (C, H2CrO4). In chromic acid the chromium is tetrahedrally coordinated by four oxygen atoms, all of which are terminal.
Details:
-
CrO2Cl2 (with Cr in +6 oxidation state) reacts with NaOH to yield a chromate salt.
-
Acidifying the chromate salt converts it to chromic acid H2CrO4.
-
In H2CrO4, the Cr atom is bonded to four oxygen atoms (all terminal).
