Question
Question: For propadiene \({H_2}C = C = C{H_2}\) , correct statement(s) is/are: A) Molecules are non planar....
For propadiene H2C=C=CH2 , correct statement(s) is/are:
A) Molecules are non planar.
B) Molecules are nonpolar.
C) Nodal plane of the pi bond formed by C1&C2; is perpendicular to that formed by C2&C3.;
D) Nodal plane of pi bond formed by C1&C2; is Coplanar to that of formed by C2&C3;
Solution
We must need to know that the allenes are in which one carbon molecule has twofold bonds with every one of its two neighboring carbon centres. Allenes are delegated cumulated dienes. The parent compound of this class is propadiene, which is itself likewise called allene. Mixes with an allene-type structure yet with in excess of three carbon particles are individuals from a bigger class of mixes called cumulenes with X=C=Y bonding.
Complete step by step answer:
We need to remember that the central atom of Allenes structures two sigma bonds and two pi bonds. The focal carbon is sp-hybridized, and the two terminal carbon molecules are sp2 hybridized. The bond point shaped by the three carbon atoms is 180∘, showing straight calculation for the focal carbon molecule. The two terminal carbon molecules are planar, and these planes are wound 90∘ from one another. The structure can likewise be seen as a "broadened tetrahedral" with a comparative shape to methane, a similarity that is preceded into the stereochemical investigation of certain subsidiary atoms.
Let us see the structure of propadiene,
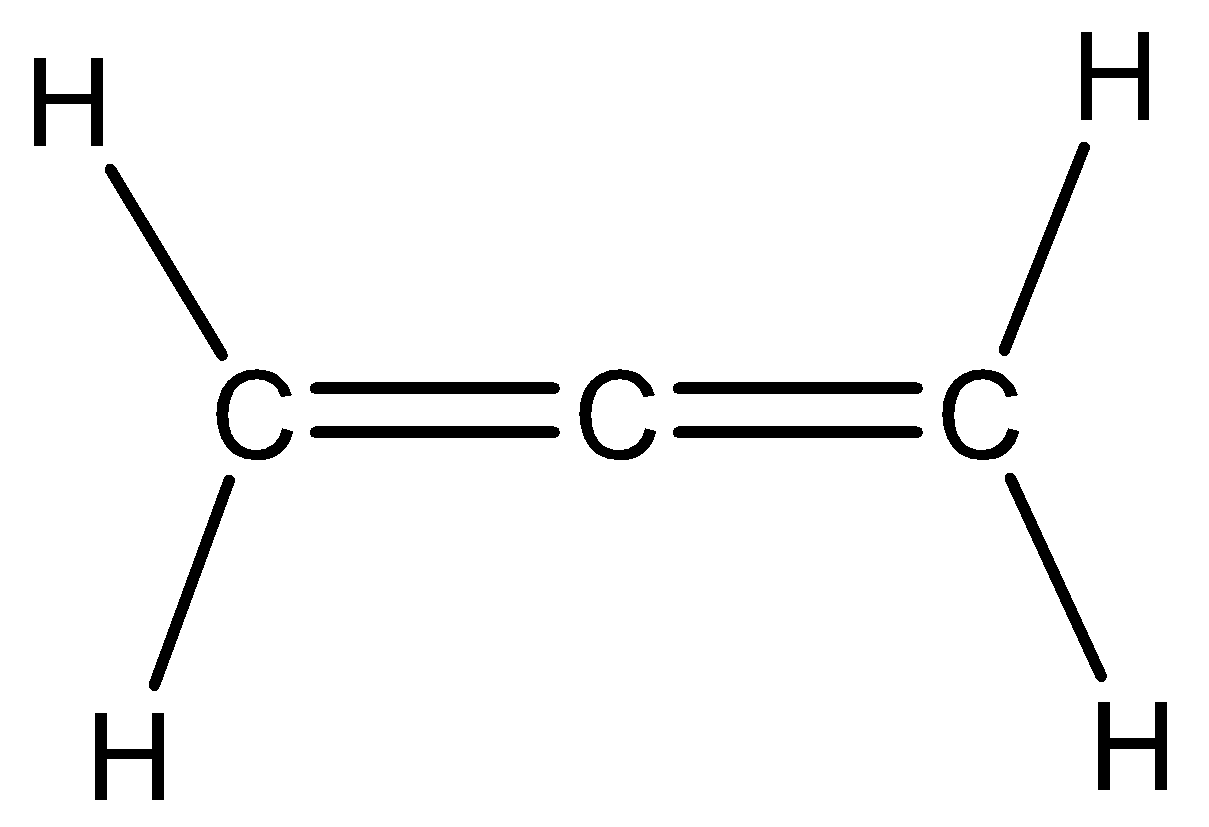
We need to know that the central atom in the above structure is sp hybridized since it has two pi bonds in addition to sigma bonds with two adjacent carbons. The remaining unhybridized p orbital is in perpendicular directions. Hence in propadiene the two methylene groups are in perpendicular planes. Hence option A and C are correct.
Note:
We must remember that hybridisation is the idea of blending nuclear orbitals into new mixture orbitals appropriate for the matching of electrons to frame compound bonds in valence bond hypothesis.
For instance, in a carbon iota which structures four single bonds the valence-shell s orbital joins with three valence-shell p orbitals to frame four comparable sp3 combinations which are masterminded in a tetrahedral plan around the carbon to cling to four distinct atoms. Hybrid orbitals are helpful in the clarification of sub-atomic math and nuclear holding properties and are evenly arranged in space. Generally half breed orbitals are shaped by blending nuclear orbitals of similar energies.
