Question
Question: For one mole of and ideal gas, the slope of \(V\)vs \(T\) curve at constant pressure of \(2atm\) is ...
For one mole of and ideal gas, the slope of Vvs T curve at constant pressure of 2atm is X Lmol−1K−1. The value of the ideal universal gas constant ′R′ in terms of X is:
A. X L atm mol−1K−1
B. 2X L atm mol−1K−1
C. 2X L atm mol−1K−1
D. 2X atm L−1 mol−1K−1
Solution
The gas constant (universal gas constant) is denoted by symbol R. It is equal to Boltzmann constant, but expressed in units of energy per temperature increment per mole i.e. the pressure- volume product, rather than energy per temperature, increment per particle.
Formula used: The ideal gas equation ⇒PV=nRT
Complete step by step answer: The gas constant occurs in the ideal gas law as follows:
PV=nRT=mRspecificT
Where, P is absolute pressure (SI units pascals), V is volume of the gas (SI unit cubic metre), n is the amount of gas (SI unit moles), m is mass (SI unit kilograms) contained in V and T is the thermodynamic temperature (SI unit Kelvins), R specific is the mass specific gas constant. The gas constant is expressed in the same physical units as molar entropy and molar heat capacity.
Rspecific =MR=Cp=Cv
M= molar mass of gas mixture
PV=nRT, or,P(nv)=RT
Or PVm=RT
(Vm=molar volume)
Vm=PRT ………. (i)
At constant pressure for 1 mol of an ideal gas, V=PRT
The relation represents a straight line passing through the equation, so, for 1 mol of an ideal gas at constant pressure, the graph of Vm vs T, will be a straight line with slope R/P.
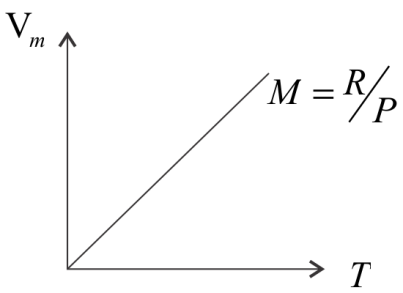
It is given in the equation that PR=m=X=given
PR=X
2atmR=X L mol−1K−1
R=2X L atm mol−1K−1.
So, the correct answer is “Option C”.
Note: The R is known as gas constant or universal gas constant or ideal gas constant. It’s value is equal to approx. 8.31446261815324J K−1mol−1.
