Question
Question: For Mn$^{3+}$ ion, the electron pairing energy P is 28000 cm$^{-1}$, $\Delta_o$ values for the compl...
For Mn3+ ion, the electron pairing energy P is 28000 cm−1, Δo values for the complexes [Mna6]3+ and [Mnb6]3− are 21000 cm−1 and 38500 cm−1 respectively. If difference in number of t2g and eg electrons in [Mna6]3+ is 'x' and difference in number of t2g and eg electrons in [Mnb6]3− is y then 10(2x + 3y) is ____
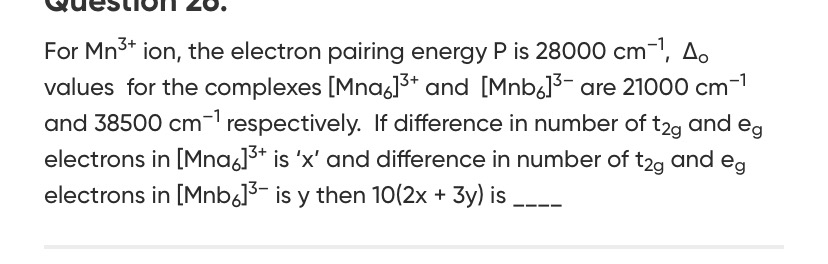
Answer
160
Explanation
Solution
-
Determine the electronic configuration of Mn3+: Manganese (Mn) has atomic number 25, with electronic configuration [Ar]3d54s2. Thus, Mn3+ has an electronic configuration of 3d4.
-
Analyze [Mna6]3+:
- Given Δo=21000 cm−1 and pairing energy P=28000 cm−1.
- Since Δo<P, the ligand 'a' is a weak field ligand, and the complex is high spin.
- For a d4 ion in an octahedral field, the high spin configuration is t2g3eg1.
- The number of t2g electrons is 3, and the number of eg electrons is 1.
- 'x' is the difference in the number of t2g and eg electrons: x=nt2g−neg=3−1=2.
-
Analyze [Mnb6]3−:
- Given Δo=38500 cm−1 and pairing energy P=28000 cm−1.
- Since Δo>P, the ligand 'b' is a strong field ligand, and the complex is low spin.
- For a d4 ion in an octahedral field, the low spin configuration is t2g4eg0.
- The number of t2g electrons is 4, and the number of eg electrons is 0.
- 'y' is the difference in the number of t2g and eg electrons: y=nt2g−neg=4−0=4.
-
Calculate the final expression:
- Substitute the values of x and y into the expression 10(2x+3y):
- 10(2×2+3×4)=10(4+12)=10(16)=160.
