Question
Question: For Manganese atom, assuming the Hund’s rule is not valid for \(l = 2\) subshell predict the unpaire...
For Manganese atom, assuming the Hund’s rule is not valid for l=2 subshell predict the unpaired electrons and electron pairs in that subshell of atom:
A) 5,1
B) 5,0
C) 1,2
D) 1,3
Solution
Hund’s rule of maximum multiplicity states that in a subshell all the orbitals are singly occupied by the electrons first and only after all the orbitals of that subshell are singly occupied, the pairing of electrons takes place.
Complete step by step answer:
The azimuthal quantum number (l) has the values 0,1,2 and 3 for the subshells s,p,d and f respectively. So, l=2 corresponds to d subshell.
As the d subshell has five orbitals and assuming that the Hund’s rule is not valid, the filling of orbitals will occur such that each orbital will accommodate two electrons first and then the next orbital will be filled.
For manganese atom, the electronic configuration is,
25Mn=[Ar]3d54s2
As per the condition, the five electrons in d subshell will be filled as follows,
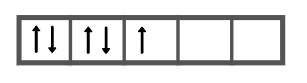
We can see that the number of unpaired electrons in manganese is 1 and the number of electron pairs is 2.
Therefore, the answer is option (C).
Note:
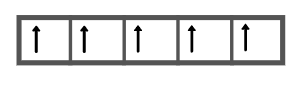
If the Hund’s rule was applicable then for l=2 subshell, that is, for d subshell the filling of orbitals would have been such that each of the orbitals would be singly occupied and only then the pairing of electrons will take place. In that case, the five ‘d’ electrons would have been filled as: