Question
Question: For He ion, the only incorrect combination is: Column \[1\]| Column \[2\]| Column \[3\] ---...
For He ion, the only incorrect combination is:
| Column 1 | Column 2 | Column 3 |
|---|---|---|
| 1s Orbital | (i)ψn,l,mla(a0Z)23e−(a0Zr) | (P)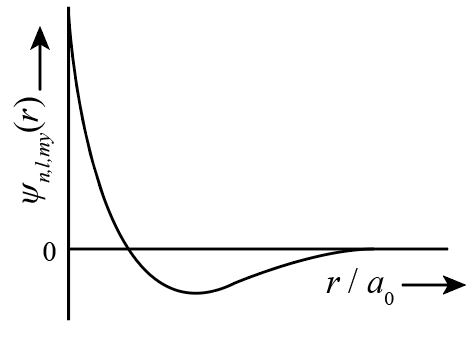 |
| 2s Orbital | (ii) one radial node | (Q) Probability density at nucleus |
| 2pz Orbital | (iii) ψn,l,mla(a0Z)25re−(a0Zr)cosθ | (R) Probability density is maximum at nucleus |
| 3dz2 Orbital | (iv) xy-plane is a nodal plane | (S) Energy needed to excite an electron from n=2 state to n=4 state is 3227 times the energy needed to excite an electron from n=2 state to n=6 state. |
A. (I) (iii) (R)
B. (II) (ii) (Q)
C. (I) (i) (S)
D. (I) (i) (R)
Solution
Helium ion is nothing but the one electron is given away form one s-orbital out of two and positive charge is acquired as we know that one electron is present in hydrogen is the same way only one electron will remain in the helium ion, hence it will behave as atom with only one electron.
Complete Step by step answer: As we know that hydrogen is the simplest atom containing only one electron. n,l,ml resembles the principle quantum number, azimuthal quantum number and magnetic quantum number respectively. Radial node is measured from n,l values. The probability density of finding the electron is never 100% because electrons are having Heisenberg uncertainty principle.
We will see the column one by one
In column 1, we will determine the radial nodes of the given orbitals by the formula
radialnode=n−l−1
For 1sorbital
⇒radialnode=1−0−1=0
For 2sorbital
⇒radialnode=2−0−1=1
For 3pzorbital
⇒radialnode=3−1−1=1
For 3dz2orbital
⇒radialnode=3−2−1=0
Now, we could see from above explanation, the radial nodes is one for 2s and 3pz orbitals but the graph for 2sorbital is shown as
By the formula
