Question
Question: For H atoms the energy required for the removal of electrons from various subshells is given. The or...
For H atoms the energy required for the removal of electrons from various subshells is given. The order of the energies would be:
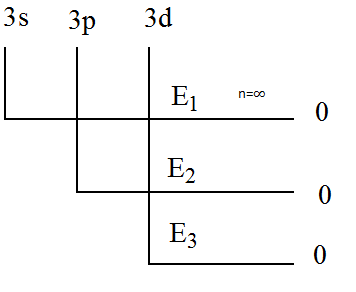
(A) E1>E2>E3
(B) E3>E2>E1
(C) E1=E2=E3
(D) None of these
Solution
We need to understand the subshells of an atom and the energy required to remove electrons from them. To proceed further, we need to understand the basic difference between shells, subshells and orbitals and how electronic configuration of atoms is the basis of them. Electrons in an atom are characterized by a set of four quantum numbers and the maximum number of electrons that can be accommodated in a shell (energy level) is based on principal quantum number (n). The maximum number of electrons that can be accommodated in a shell is calculated by the formula 2n2 where the shell number.
Complete step by step answer:
Given in the question that the energy required for the removal of electron from subshell 3s is E1 , for the removal of electron from subshell 3p is E2 and for the removal of electron from subshell 3d is E3 .
It is clear that the principal quantum number of all the subshells is 3. In other words, 3 is the shell number.
The most probable distance between the nucleus and the electrons is described by the shell number or principal quantum number. A larger value of the principal quantum number implies a greater distance between the electron and the nucleus and hence lesser force will be required to remove the electron from it.
Electrons belonging to the same shell will require the same amount of energy to remove them from their respective shells as they are equidistant from the nucleus.
Hence, the electrons present in the 3rd shell will have the same energy and s, p and dare subshells of the same shell. Therefore, E1=E2=E3.
Hence the correct option is option (C).
Additional information:
The understanding of differences between shells, subshells and orbitals will help to write the electronic configuration of any atom with a given atomic number. Furthermore, the distribution of electrons to write the electronic configuration follows certain rules. If two electrons are filled in the ‘ s ’ subshell of the first shell, the electronic configuration is noted as 1s2 where n=1 which is the principal quantum number.
Note:
It must be noted that the energies of electrons in nth shell can be calculated as Enth=−n213.6eV, where eV is the SI unit of energy and the minus sign depicts that the energy is calculated for an electron. Hence for the given question, electrons in the 3s, 3p and 3d orbitals will have the same energies as the value of n is the same, i. e. 3.
