Question
Question: For \[{{H}_{3}}P{{O}_{3}}\]and \[{{H}_{3}}P{{O}_{4}}\] the correct choice is: A. \[{{H}_{3}}P{{O}_...
For H3PO3and H3PO4 the correct choice is:
A. H3PO3 is dibasic and reducing.
B. H3PO3 is dibasic and non-reducing.
C. H3PO4 is tribasic and reducing.
D. H3PO4 is tribasic and non-reducing.
Solution
Hint: Phosphoric acids are strong acids. It has 3 hydrogen atoms but only 2 are isonisable. Thus it is soluble in water.
Complete step by step solution:
Phosphorus acid or phosphonic acid is a colourless deliquescent crystalline solid which is highly acidic in nature. Even though it has 3 hydrogen, only 2 are ionisable. Hence it is dibasic acid and ionises as
H3PO3→H++H2PO3−
H3PO3−→H++H2PO32−
The other hydrogen, which does not take part in ionisation, has a reducing nature. Thus H3PO3 act as a reducing agent. So the correct answer for the question is (a).
-Phosphorus acid is obtained by hydrolysis of phosphorus trichloride and phosphorus trioxide.
-Phosphorus acid and its salts are strong reducing agents, as they are readily oxidisable to phosphoric acid and phosphates, respectively.
HPO32−+3OH−→PO43−+2H2O+2e−
- Phosphorus acid has the capability of reducing salts of copper, silver, gold etc., to their respective metals.
-The structure of phosphorus acid is shown as
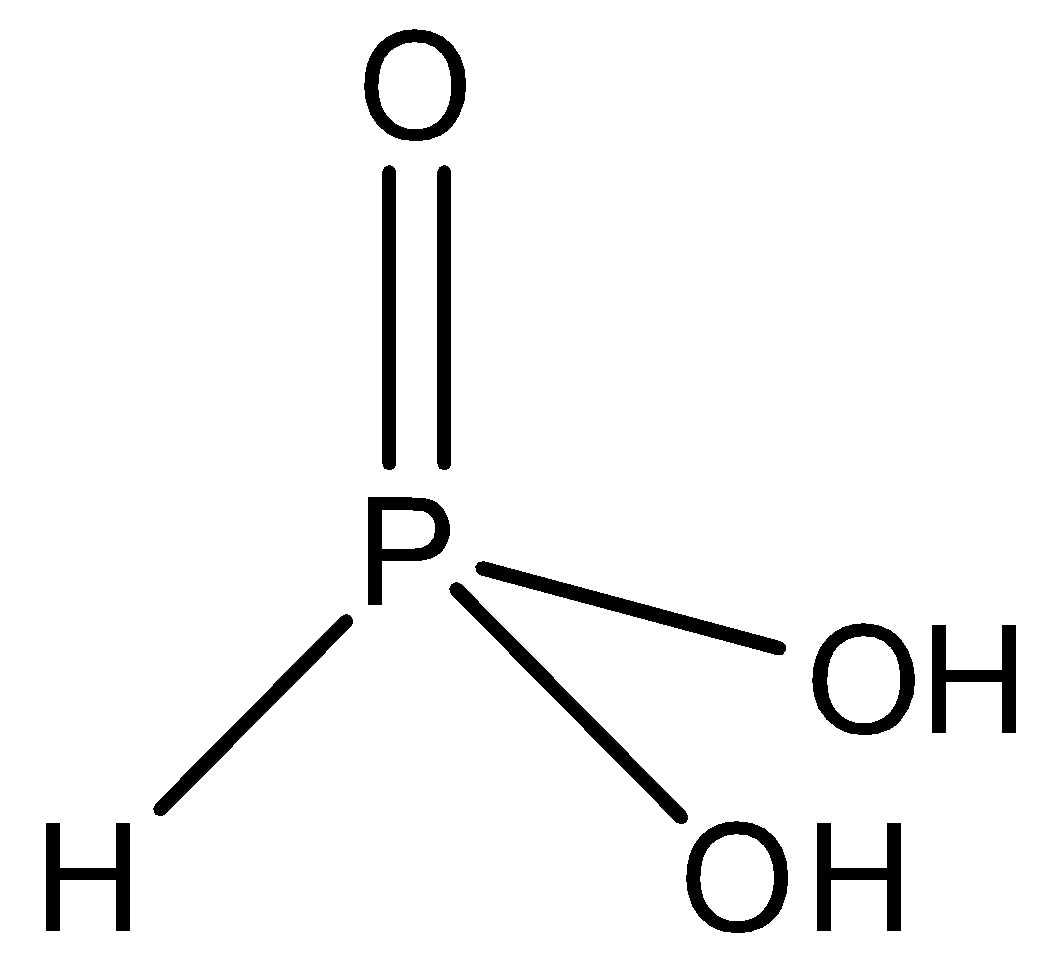
-Orthophosphoric acid H3PO4 is a tribasic weak acid. It also has 3 hydrogen atoms, but has 4 oxygen atoms. It is not a reducing agent because all the 3 of its hydrogen ionises in water. It structure is shown as
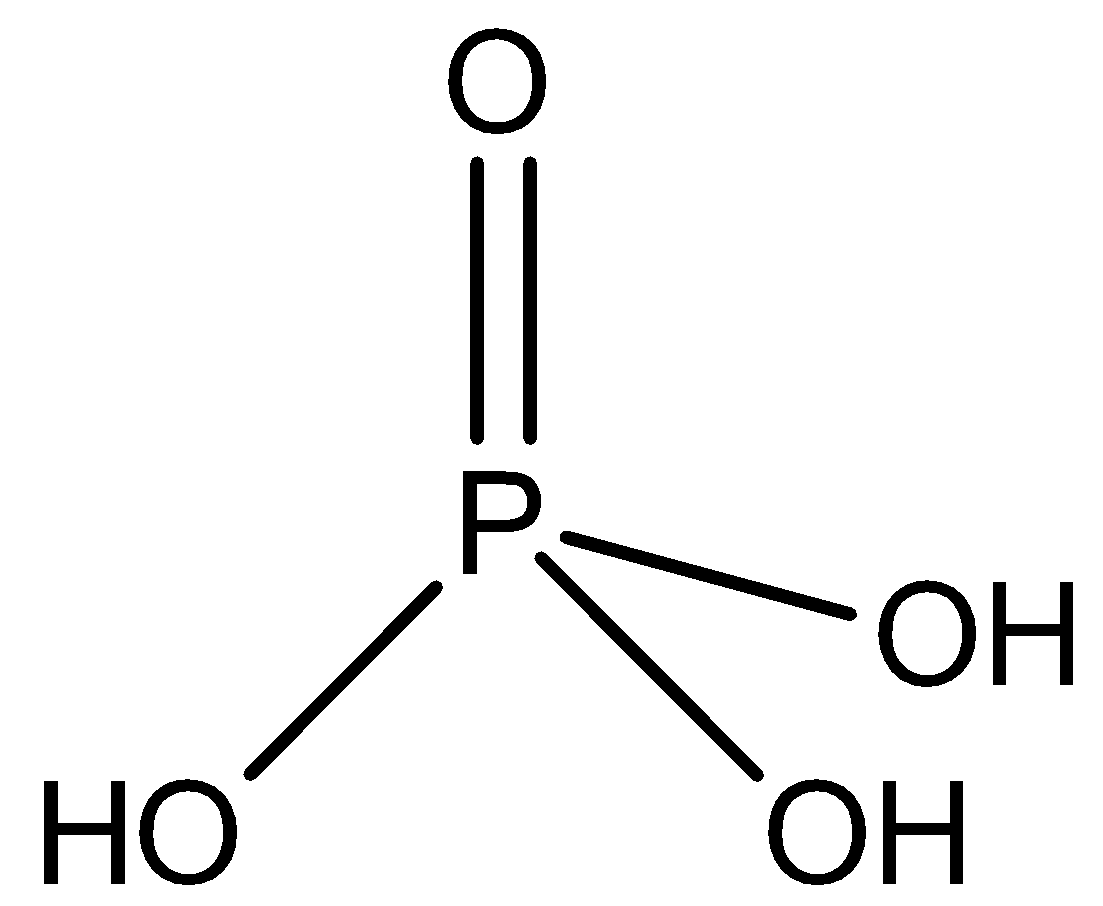
-Phosphoric acid is a syrup liquid which combines with water gives a semi hydrate 2H3PO4.H2O
-It ionises in 3 stages
H3PO4⇌H++H2PO4−
H3PO4−⇌H++H2PO42−
H3PO42−⇌H++PO43−
Its first ionisation takes place quite fast, but the second and third ionisation takes place very slow. It forms a series of salts.
Thus, the correct answer is option (a), Phosphorus acid is dibasic and reducing.
Note: There are chances that we assume even phosphoric acid is reducing like phosphorus acid. But it is not a reducing agent.
