Question
Question: For given solution in the questions molarity can be treated as molality and solute(s) is/are nonvola...
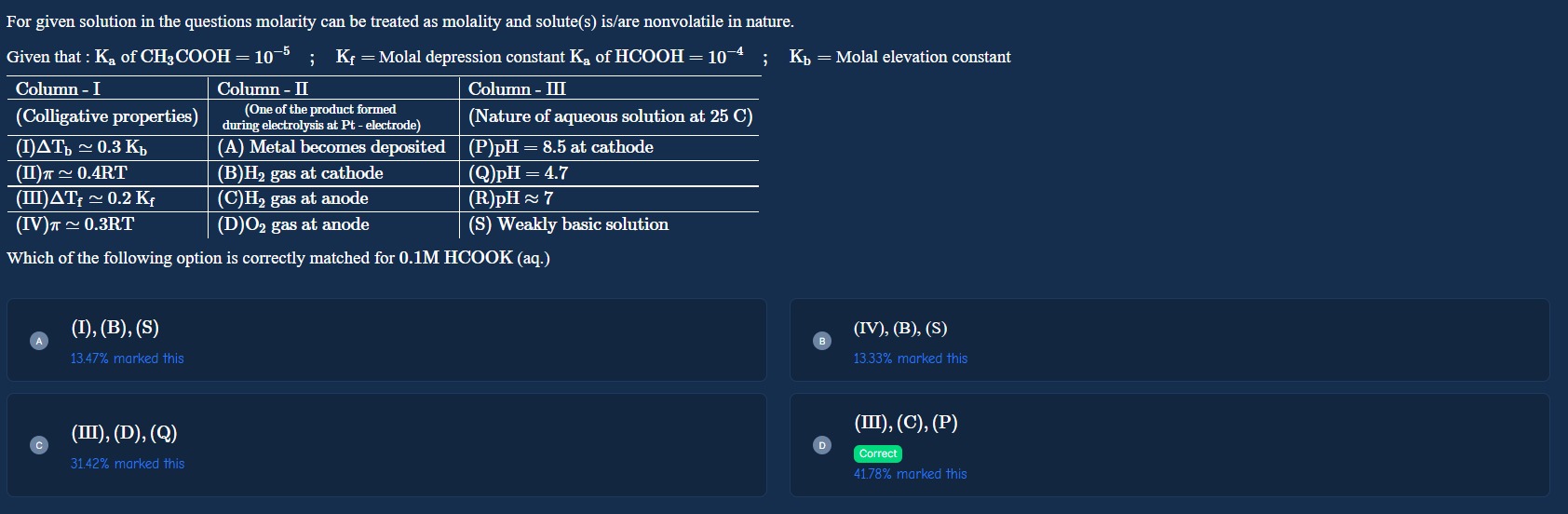
For given solution in the questions molarity can be treated as molality and solute(s) is/are nonvolatile in nature.
Given that : Ka of CH3COOH=10−5 ; Kf = Molal depression constant Ka of HCOOH=10−4 ; Kb = Molal elevation constant
| Column - I (Colligative properties) | Column - II (One of the product formed during electrolysis at Pt - electrode) | Column - III (Nature of aqueous solution at 25 C) |
|---|---|---|
| (I) ΔTb≈0.3Kb | (A) Metal becomes deposited | (P) pH = 8.5 at cathode |
| (II) π≈0.4RT | (B) H2 gas at cathode | (Q) pH = 4.7 |
| (III) ΔTf≈0.2Kf | (C) H2 gas at anode | (R) pH ≈ 7 |
| (IV) π≈0.3RT | (D) O2 gas at anode | (S) Weakly basic solution |
Which of the following option is correctly matched for 0.1M HCOOK (aq.)
A
(I), (A), (P)
B
(II), (B), (Q)
C
(III), (C), (P)
D
(IV), (D), (S)
Answer
(III), (C), (P)
Explanation
Solution
0.1M HCOOK (aq.) will have:
- ΔTf≈0.2Kf (Molal depression constant)
- H2 gas at cathode (Product formed during electrolysis at Pt - electrode)
- pH = 8.5 at cathode (Nature of aqueous solution at 25 C)
